A 36-year-old man with congenital afibrinogenemia and a previous left middle cerebral artery (MCA) stroke presented to the hospital with new headache, binocular diplopia and vertigo. He was taking clopidogrel with reported good adherence, but while he was scheduled to receive fibrinogen concentrate weekly his adherence was poor. Neurological examination revealed restricted right eye abduction and elevation. Right hemiparesis, dysarthria, impaired gait and upper motor neuron findings could not be differentiated from the residual deficits of his prior stroke. CT of the head only showed the known previous left MCA stroke. MRI confirmed new strokes. Diffusion-weighted imaging showed multiple acute infarcts with restricted diffusion in the superior right cerebellar hemisphere, inferior right cerebellar hemisphere, cerebellar vermis, left cerebellar hemisphere, left occipital lobe and right parietal lobe (Figure 1A). These regions appeared hypointense on apparent diffusion coefficient (Figure 1B), suggesting that they were due to acute infarction. The right superior cerebellar artery was irregular distally on the CT angiogram, but otherwise no new relevant vessel anomalies were reported. The other older vessel anomaly is chronic occlusion of the left internal carotid artery with tandem occlusion of the left mid-to-distal M1 MCA, but there was no acute branch vessel occlusion seen otherwise in this region. Transthoracic echocardiogram was unremarkable; the patient did have surgical repair of a patent foramen ovale at least 8 years before this presentation, but the exact date of the repair is unknown. As the patient’s fibrinogen level was low at 0.3 g/L (normal 1.9-4.1 g/L), he received the fibrinogen concentrate. As new infarcts occurred while the patient was on clopidogrel, apixaban (5 mg twice daily) was started with the intent for him to remain on lifelong anti-coagulation.
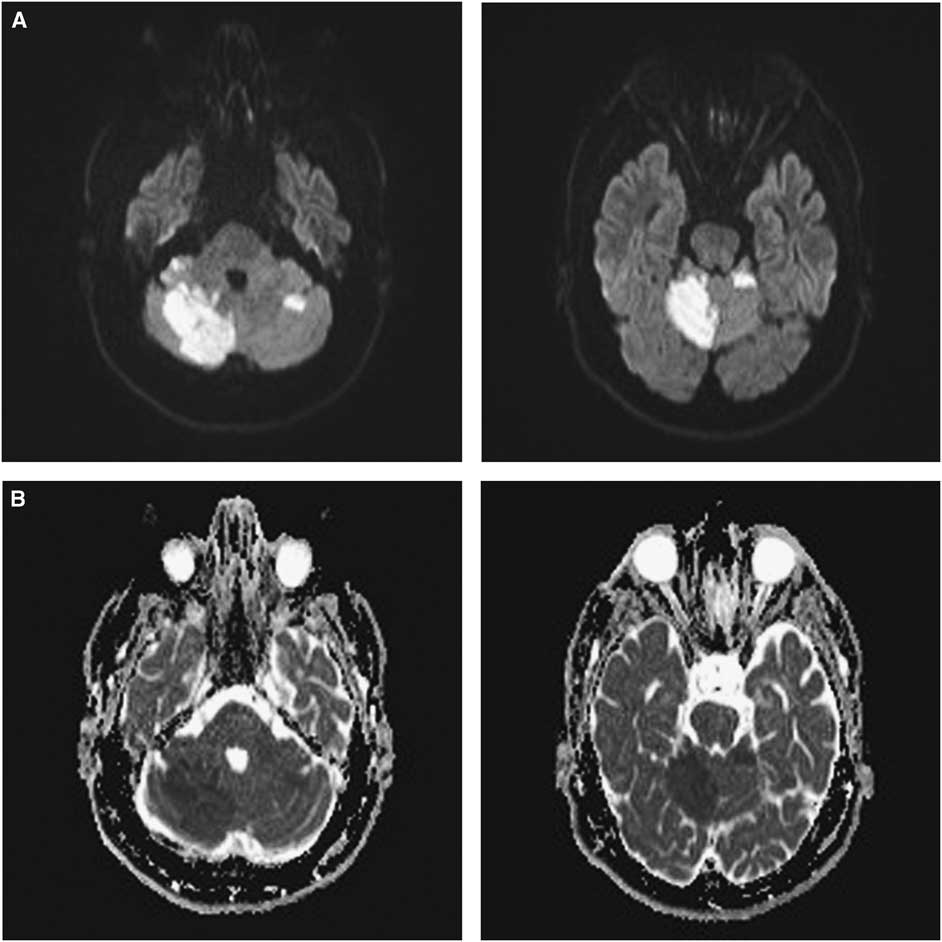
Figure 1 Multiple acute brain infarcts in a patient with congenital afibrinogenemia. (A) Diffusion-weighted imaging (DWI) showing diffusion restriction in the right and left cerebellar hemispheres and the cerebellar vermis. (B) Corresponding apparent diffusion coefficient showing hypointensities in the same regions with diffusion restriction on DWI, supporting that they are due to acute infarction.
Our patient’s congenital afibrinogenemia was diagnosed by functional and immunologic assays at the age of 5 years when work-up for easy bruising identified a prolonged prothrombin time and activated partial thromboplastin time. Fibrinogen studies then revealed his clottable fibrinogen level to be low at 0.06 g/L, and his immunoreactive fibrinogen was low both by latex immunoassay at <0.01 g/L and by Laurell immunoelectrophoretic assay at <0.04 g/L. Genotyping showed compound heterozygous mutations—(a) FGA: c.117delT, p.(Val40TrpfsTer31) (frameshift mutation affecting fibrinogen subunit A) and (b) FGA: c.510+1G>T (splice variant affecting fibrinogen subunit A). His parents and three siblings were phenotypically unaffected.
Several previous bleeding and clotting events had occurred, including a retroperitoneal bleed and right-leg deep-vein thrombosis (DVT). The DVT was to be treated with tinzaparin for 6 months and weekly fibrinogen infusions, but the patient did not adhere to the recommendations. He also had a left MCA stroke nearly 15 years ago.Reference Chun, Poon, Haigh, Seal, Donahue and Royston 1 At that time, a hypercoagulability work-up was negative. It included testing for antithrombin III, protein C and S deficiency, factor V Leiden, prothrombin G20210A mutation and anti-phospholipid syndromes (lupus-type inhibitor, anti-cardiolipin antibody and anti-β2-glycoprotein 1-antibodies). However, vegetations of the aortic valve leaflets were discovered. During open-heart surgery, the leaflets were noted to be of normal morphology. Fibrinous material present at the commissure of the left and right aortic leaflets was debrided.Reference Chun, Poon, Haigh, Seal, Donahue and Royston 1 Surgical management was complicated by further thrombotic events: intra-operatively he had acute left main coronary artery thrombosis, and post-operatively he developed catheter-related DVT involving bilateral subclavian and axillary veins with extension into the superior vena cava. He was treated with low-molecular-weight heparin for 8 months.
Congenital afibrinogenemia is an autosomal recessive condition with a prevalence of 1 in 1,000,000.Reference Casini, de Moerloose and Neerman-Arbez 2 Normally, fibrinogen is converted to fibrin to produce the cross-linked fibrin clot; its absence is associated with a propensity to bleed. Bleeding can occur anywhere, and cerebral bleeds can be a major cause of death.Reference Casini, de Moerloose and Neerman-Arbez 2 Thrombotic events can also occur. One proposed mechanism of thrombosis is that fibrin binds thrombin and is historically called antithrombin I. Without fibrin binding, thrombin levels increase and become involved in platelet activation and aggregation.Reference Santoro, Massaro and Venosi 3 Although platelet aggregation is normally mediated by fibrinogen, platelet aggregation may occur through von Willebrand factor binding in the absence of fibrinogen.Reference Santoro, Massaro and Venosi 3 Most thrombotic events in patients with congenital afibrinogenemia involved leg arteries,Reference Santoro, Massaro and Venosi 3 but ischemic stroke has been reported. Vertebral artery dissection has been treated with heparin after fibrinogen replacement,Reference Garcia-Monco, Fernandez Canton and Gomez Beldarrain 4 , Reference Laufs, Weidauer, Heller, Lorenz and Neumann-Haefelin 5 and internal jugular vein thrombosis has been treated with rivaroxaban.Reference Margaglione, Vecchione and Cappucci 6
There are no guidelines regarding management of stroke in congenital afibrinogenemia. It is unclear whether correcting the fibrinogen deficiency prevents a hypercoagulable state as there appears to be no relationship between fibrinogen replacement and thrombosis.Reference Bornikova, Peyvandi, Allen, Bernstein and Manco-Johnson 7 Our patient has experienced multiple serious thrombotic events with no obvious relationship to him receiving fibrinogen replacement. It is also unclear whether a patient with congenital afibrinogenemia who has an ischemic stroke should be on lifelong anti-coagulation. Furthermore, the anti-coagulant selected should enable accurate monitoring of fibrinogen levels. Direct thrombin inhibitors (e.g. dabigatran) can make levels of fibrinogen appears falsely low,Reference Castellone and Van Cott 8 whereas direct factor Xa inhibitors (e.g. apixaban, rivaroxaban) do not interfere with the fibrinogen assay, making them better options. As our patient is likely to have failed anti-platelet therapy, it seemed prudent to initiate lifelong anti-coagulation. Unfortunately, this man had a cerebellar hemorrhage requiring surgical evacuation 23 months after this reported stroke. He had not been adherent with either his apixaban or his fibrinogen infusions suggesting the hemorrhage reflected the natural history of the disease. Measures to improve his adherence failed. He recovered well but given his poor treatment adherence anti-coagulation was discontinued. Best management of this challenging situation remains unclear.
Disclosures
The authors do not have anything to disclose.
Statement of Authorship
NN drafted the manuscript. NR, M-CP, and LMM were involved with the interpretation of the study results, and critically reviewed the manuscript. All authors approved the final version of the manuscript.



