Introduction
Endovascular thrombectomy (EVT) is the standard of care for patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO). Reference Powers, Rabinstein and Ackerson1 Efficacy is dependent on timely recanalization of the occluded artery. Despite significant improvements in clinical outcomes with EVT, more than half of patients remain functionally dependent (as defined by a modified Rankin scale (mRS) score of greater than two) 3 months after their initial stroke. Reference Goyal, Menon and van Zwam2 It may be that additional factors, such as anesthetic strategy, influence both the technical success of the procedure and overall outcomes.
The optimal anesthetic strategy for those undergoing EVT is controversial; at present, GA is routinely used at some centers, used only for specific patient types or situations or eschewed completely. Options include local anesthetic (LA) use at the arterial access site only, conscious or procedural sedation (CS) without intubation, or general anesthesia (GA) with full airway control and optional use of neuromuscular blockade. The distinction between CS and GA is generally drawn at the use of intubation for full airway control.
Proposed advantages and disadvantages associated with each type of anesthesia are shown in Table 1. Conventionally, GA has been widely used in neuro-intervention for elective endovascular procedures such as the coiling of intracranial aneurysms. Particularly, distally in the intracranial circulation, the complete absence of movement has been widely considered essential for both technical procedural success and to minimize complications and therefore a major advantage of GA.
Table 1: Advantages and disadvantages by anesthetic strategy
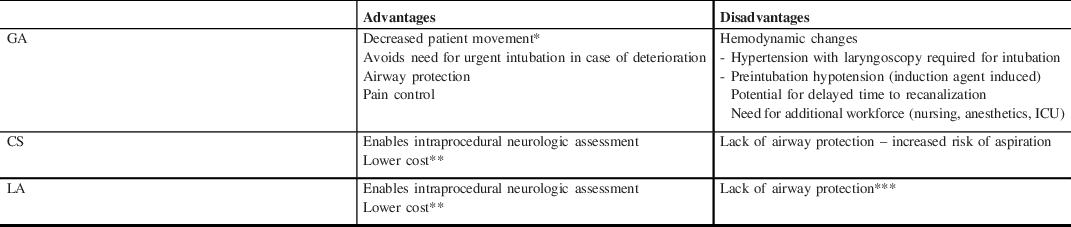
CS=conscious sedation; GA=general anesthesia; ICU=intensive care unit; LA=local anesthetic.
* Theoretically increasing chance of successful recanalization and reducing risk of distal embolization and vessel perforation/dissection.
** Less medication, monitoring, staffing, need for ICU admission compared with GA.
*** Lower risk of aspiration compared with CS.
Initial nonrandomized studies evaluating anesthetic choice suggested an association with adverse outcomes and GA. Reference Sugg, Jackson, Holloway, Martin, Akhtar and Rymer3–Reference Pop, Severac and Happi Ngankou18 A post hoc analysis of MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in The Netherlands), in which sites prespecified their anesthetic technique prospectively, demonstrated that the beneficial effect of EVT on clinical outcomes was nullified in those treated with GA. Reference Berkhemer, van den Berg and Fransen19 The HERMES (Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke) collaboration meta-analysis reported that patients treated with GA had poorer outcomes compared with those managed without GA; the benefit of EVT on functional outcomes was reduced but still observable. Reference Campbell, van Zwam and Goyal20 More recently, five single center RCTs have published contrasting results, reporting either improved or no significant difference in clinical outcomes according to anesthetic strategy. Reference Simonsen, Yoo and Sørensen21–Reference Schönenberger, Uhlmann and Hacke25 This stark contrast between larger multicenter cohort studies showing a negative association between GA use and outcomes and smaller single center randomized trials showing improved or no difference in outcomes with GA is the fundamental source of ongoing controversy in the field.
Different anesthetic strategies have variable effects on cerebral and systemic hemodynamics, revascularization times, and periprocedural complications (see Figure 1). Interpretation of results from nonrandomized studies is affected by confounding by indication and selection bias. Patients with more severe stroke and multiple comorbidities, the very factors that predict poorer outcome overall, are more likely to be managed under GA. Anesthetic practices in the HERMES trials varied substantially, in contrast with the highly specified protocols of the most recent anesthesia-specific RCTs.

Figure 1: Anesthesia and EVT – key variables.
The objective of this review is to examine the influence of different anesthetic management strategies on clinical outcomes in those with AIS due to LVO of the anterior circulation, with secondary consideration of potential underlying mechanisms.
Clinical Results
Retrospective Studies
Observational, predominantly retrospective studies, which have attempted to evaluate the association between anesthetic strategy and clinical outcomes among patients with AIS managed with EVT, have reported mixed results (see Table 2). CS has been associated with improved functional outcomes Reference Sugg, Jackson, Holloway, Martin, Akhtar and Rymer3–Reference Feil, Herzberg and Dorn14 and reduced mortality Reference Abou-Chebl, Lin and Hussain4–Reference McDonald, Brinjikji, Rabinstein, Cloft, Lanzino and Kallmes7,Reference Just, Rizek, Tryphonopoulos, Pelz and Arango9,Reference Powers, Dornbos and Mlynash13,Reference Feil, Herzberg and Dorn14 compared with GA; others have reported no difference in outcomes between CS and GA. Reference Li, Deshaies and Singla26–Reference Byrappa, Lamperti, Ruzhyla, Killian, John and St Lee31 LA has similarly been associated with better functional outcomes compared with GA, Reference Davis, Menon and Baghirzada15–Reference Pop, Severac and Happi Ngankou18 often with reduced mortality, Reference Davis, Menon and Baghirzada15,Reference Abou-Chebl, Yeatts and Yan16 although these findings are contrasted by those from a subanalysis of Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke Reference Bracard, Ducrocq and Mas32 and a prospective study by Wu et al. Reference Wu, Jadhav and Zhao33 Only two studies have directly compared outcomes in those managed with CS versus LA. Work from 1034 patients in the Endovascular Treatment in Ischemic Stroke registry in France reported higher rates of good functional outcome with CS versus LA (mRS 0–2 at 3 months CS 52% vs LA 40%, p = 0.028). Reference Benvegnù, Richard and Marnat34 A retrospective review from one of the MR CLEAN centers reported contrasting results (mRS 0–2 at 3 months CS 22% vs LA 47%, OR 0.4 [0.2–0.8]). Reference van de Graaf, Samuels and Mulder35 Both Goldhoorn et al. and Cappellari et al. retrospectively evaluated outcomes in patients with LA, CS, or GA. In both studies, those managed with LA had improved functional outcomes (Goldhoorn mRS 0–2 GA 35%, CS 25%, LA 41%, p ≤ 0.01; Cappellari mRS 0–2 GA 42.5%, CS 46.6%, LA 52.4%, p ≤ 0.001) and reduced mortality (Goldhoorn GA 32%, CS 36%, LA 27%, p = 0.04; Cappellari GA 21.5% CS 19.7% LA 14.8%, p ≤ 0.001). Reference Goldhoorn, Bernsen and Hofmeijer36,Reference Cappellari, Pracucci and Forlivesi37 The range of rates of good outcome among these various studies is large implying likely differences in the populations under study. Interpretation of results from these nonrandomized studies is limited (inherent bias in trial design, combination of LA and CS into single comparator group); however, findings suggest improved outcomes in those managed without GA and point toward better outcomes in patients managed with LA rather than CS. In addition to improved outcomes, patients managed without GA have a shorter length of stay as reported by powers (GA 8.02 days (5.35–12.18 days), CS 5.93 days (3.31–8.85 days), p = 0.03), Reference Powers, Dornbos and Mlynash13 and Bekelis et al. (GA 19.6 days, CS 11.7 days, unadjusted difference 7.9 (CI 5.1–10.7) Reference Bekelis, Missios, MacKenzie, Tjoumakaris and Jabbour38 and a reduced cost of hospitalization (GA $USD 34,903 ($25,530–55,444), CS $26,775 ($18,790–39,935), p ≤ 0.0001). Reference McDonald, Brinjikji, Rabinstein, Cloft, Lanzino and Kallmes7
Table 2: Nonrandomized studies
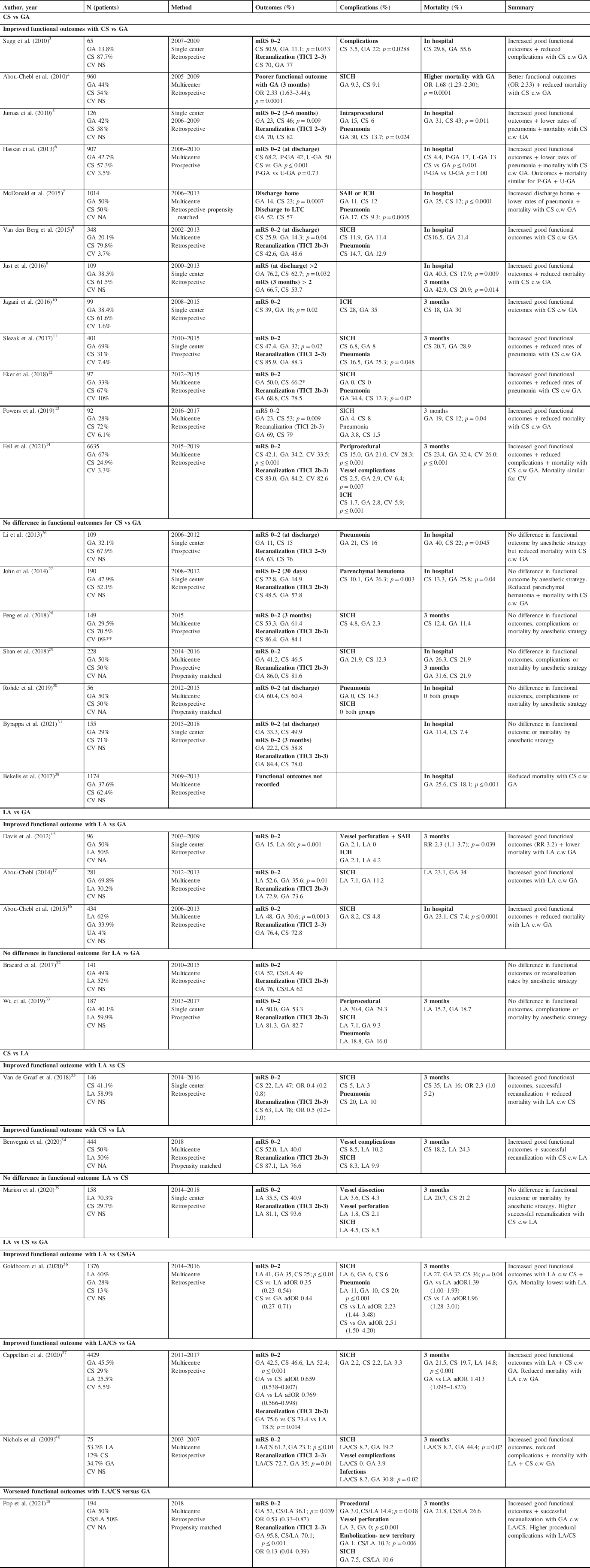
adOR=adjusted odds ratio; CS=conscious sedation; CV=conversion from LA or CS to GA; GA=general anesthetic; ICH=intracranial hemorrhage; LA=local anesthesia; MAC=minimum alveolar concentration; mRS=modified Rankin scale; NA=nonapplicable – propensity matched; NS=not stated; OR=odds ratio; P-GA=planned GA; SICH=symptomatic intracranial hemorrhage; TICI=thrombolysis in cerebral infarction; UA=undetermined anesthesia; U-GA=unplanned GA.
mRS scores recorded at 3 months unless otherwise stated. Only significant p values provided.
* GA associated with lower rate of functional independence when mRS corrected for differences in baseline characteristics (OR 0.32; p = 0.05).
**T hese patients were excluded.
Anesthesia-Specific RCTs
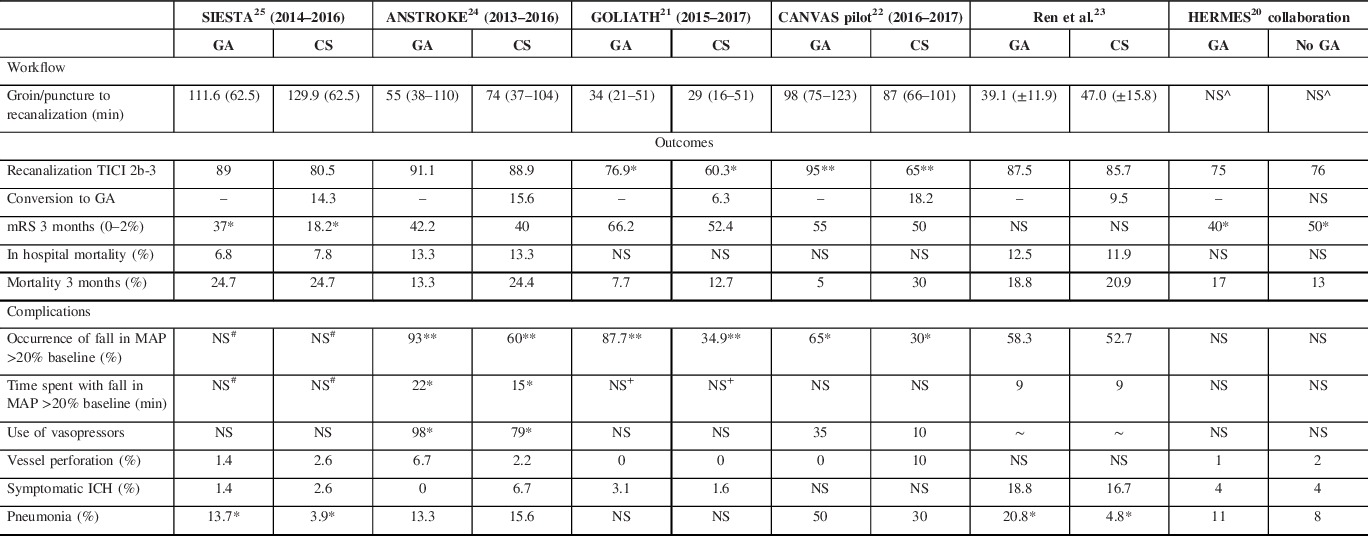
Five single center RCTs have examined the influence of anesthetic strategy on outcomes among patients with AIS due to anterior circulation LVO (see Tables 3–5). Overall rates of functional independence (mRS 0–2 at 90 days) were variable, ranging from 27.3% in SIESTA Reference Schönenberger, Uhlmann and Hacke25 to 59.4% in GOLIATH. Reference Simonsen, Yoo and Sørensen21 Similar variability was seen in the HERMES trials, with the most comparable (by anesthetic strategy) reporting functional independence in 32.6% patients in MR CLEAN (38% GA), Reference Berkhemer, Fransen and Beumer41 60.0% in Solitaire With the Intention For Thrombectomy as PRIMary Endovascular Treatment (37% GA), Reference Saver, Goyal and Bonafe42 and 71.0% in Extending the Time for Thrombolysis in Emergency Neurological Deficits – Intra-Arterial (36% GA). Reference Campbell, Mitchell and Kleinig43 Only two of the anesthesia-specific RCTs, ANSTROKE and the work by Ren et al., specifically evaluated the impact of anesthetic outcome on functional status 3 months poststroke. Reference Ren, Xu, Liu, Liu, Wang and Gao23,Reference Löwhagen Hendén, Rentzos and Karlsson24
Table 3: Anesthesia-specific RCTs – baseline characteristics
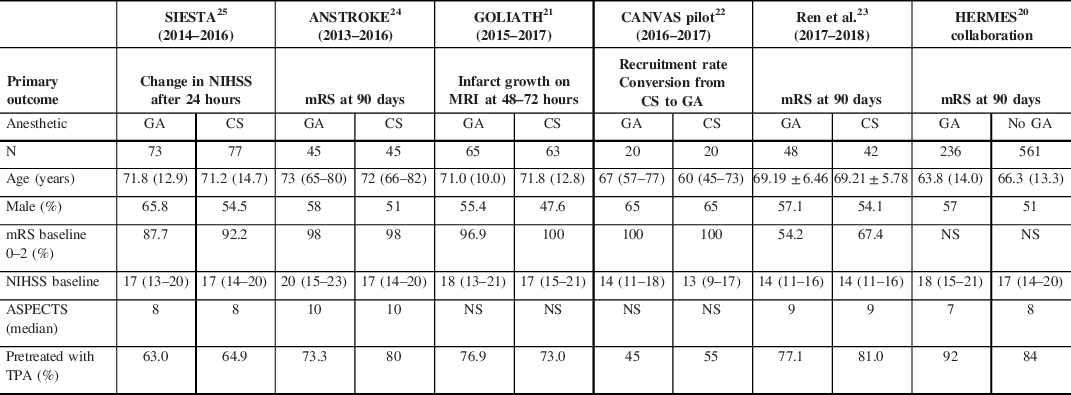
ANSTROKE=Anesthesia During Stroke Trial; ASPECTS=Alberta Stroke Programme Early CT Score; CANVAS=Choice of ANesthesia for EndoVAScular Treatment of Acute Ischemic Stroke; GOLIATH=General Or Local anesthesia in Intra Arterial Therapy; HERMES=Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke trials; mRS=modified Rankin scale; NIHSS=National Institutes of Health Stroke Scale; NS=not stated; SIESTA=Sedation versus Intubation for Endovascular Stroke Treatment; TPA=Tissue Plasminogen Activator.
Table 4: Anesthesia-specific RCTs – anesthetic protocols
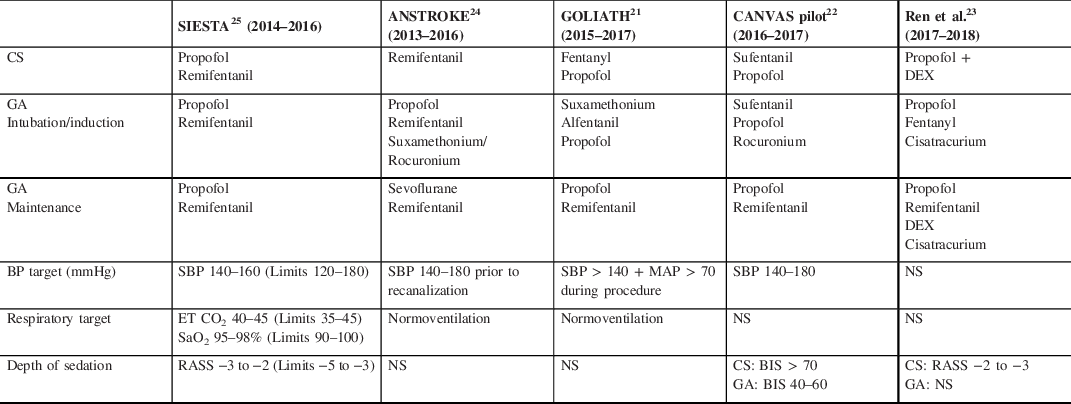
BIS=bispectral index; CS=conscious sedation; DEX=dexmedetomidine; ET CO2=end tidal carbon dioxide; GA=general anesthetic; MAP=mean arterial pressure; NS=not stated; RASS=Richmond Agitation Sedation Scale; SaO2=arterial oxygen saturation; SBP=systolic blood pressure.
Table 5: Anesthesia-specific RCTs – outcomes
ANSTROKE=Anesthesia During Stroke Trial; CANVAS=Choice of ANesthesia for EndoVAScular Treatment of Acute Ischemic Stroke; GA=general anesthesia; GOLIATH=General Or Local anesthesia in Intra Arterial Therapy; HERMES=Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke trials; MAP=mean arterial pressure; mRS=modified Rankin scale; NS=not stated; SIESTA=Sedation versus Intubation for Endovascular Stroke Treatment; TICI=thrombolysis in cerebral infarction.
*p ≤ 0.05, **p ≤ 0.001.
# SIESTA reported critical hypo (SBP < 120 mmHg) and hypertension (SBP > 180 mmHg) rather than MAP values.
+GOLIATH used time MAP < 70 mmHg as similar index.
^HERMES reported onset to randomization and randomization to reperfusion rather than puncture to recanalization.
∼Ren et al. reported vasopressor use for each individual agent. For CS range 11.9%–23.8%. For GA range 6.25%–22.9%. No significant difference between groups.
SIESTA (Sedation versus Intubation for Endovascular Stroke Treatment) randomized 150 patients (73 GA, 77 CS) from the University of Heidelberg in Germany. GA was the standard of care prior to trial commencement; CS was the intervention in the trial. The CS protocol, implemented 6 months prior to study commencement, targeted a Richmond Agitation Sedation Scale of −3 to −2. There was no difference in primary outcome, change in NIHSS at 24 hours, between groups (GA mean −3.2, CS mean −3.6, difference −0.4 (−3.4–2.7) p = 0.82), although patients managed with GA were significantly more likely to achieve functional independence at 3 months (GA 37%, CS 18.2%, difference −18.8 (−32.8 to −4.8), p = 0.01). Reference Schönenberger, Uhlmann and Hacke25
ANSTROKE (Anesthesia During Stroke Trial) randomized 90 patients (45 GA, 45 CS) from Sahlgrenska University Hospital in Sweden who presented within 8 hours of onset. GA was considered the intervention, suggesting CS was the standard of care prior to trial enrollment. Neither the primary endpoint of percentage of patients that achieved an mRS of 0–2 at 3 months (GA 42.2%, CS 40%, p = 1.00) nor the median mRS at 3 months (GA 3 (1–4), CS 3 (1–5.5), p = 0.5001) differed by anesthetic strategy. Reference Löwhagen Hendén, Rentzos and Karlsson24
In GOLIATH (General Or Local anesthesia in Intra Arterial Therapy), both GA and CS were routinely used at the Aarhus University Hospital, Denmark prior to trial commencement. Similar to ANSTROKE, no target level of sedation was provided in the trial protocol. The primary outcome measure, infarct growth on MRI at 48–72 h, did not differ by anesthetic strategy (GA 8.2 ml (2.2–38.6), CS 19.4 ml (2.4–79.0), p = 0.10), although final infarct volume was lower in those managed with GA (GA 22.3 ml (8.1–64.5), CS 38.0 ml (16.7–128.0), p = 0.04), potentially as a result of higher successful recanalization rates (TICI 2b-3) with GA (GA 76.9%, CS 60.3%, p = 0.04). Those managed with GA had better functional outcomes at 3 months (mRS 0–2 GA 66.2%, CS 52.4%; median mRS GA 2 (1–3), CS 2 (1–4), p = 0.04). Reference Simonsen, Yoo and Sørensen21
The CANVAS (Choice of ANesthesia for EndoVAScular Treatment of Acute Ischemic Stroke) trial assessed recruitment rates and rates of conversion from CS to GA to facilitate a larger trial evaluating the influence of anesthetic strategy on clinical outcomes (in progress). The target level of sedation in the CS group was a bispectral index of 70 or more, so that patients maintained normal, purposeful responses to stimuli, and were able to protect their own airway. Only 31.4% of patients who presented to Beijing Tiantan Hospital Capital Medical University with LVO were enrolled in the trial (40 patients, GA 20, CS 20); the most common reason for exclusion was LVO of the posterior circulation. A fifth (18.2%) of CS patients were converted to GA. Functional outcomes at 3 months were similar across groups (mRS 0–2 GA 55%, CS 50%), although mortality was higher with CS (GA 5%, CS 30%). Reference Sun, Liang and Wu22
Most recently, Ren et al. randomized 90 patients (GA 48, CS 42), using the same depth of CS as SIESTA and demonstrated no difference in outcomes by anesthetic strategy (median mRS at discharge 2 for GA and CS, p = 0.890; mRS 3 months 2.5 for GA and CS, p = 0.796). Reference Ren, Xu, Liu, Liu, Wang and Gao23
Rates of symptomatic ICH (SICH) did not differ by anesthetic strategy in the anesthesia-specific RCTs, weakening the argument that GA is necessary to prevent excessive patient movement to reduce the risk of vessel perforation. With the exception of Ren et al., rates of SICH in the anesthesia-specific RCTs were comparable to results from the HERMES collaboration (SICH Ren et al. GA 18.8%, CS 16.7%; HERMES GA 4%, CS 4%). Reference Campbell, van Zwam and Goyal20,Reference Ren, Xu, Liu, Liu, Wang and Gao23 GA was associated with higher rates of pneumonia in SIESTA (GA 13.7%, CS 3.9%, p = 0.03) Reference Schönenberger, Uhlmann and Hacke25 and the work by Ren et al. (GA 20.8%, CS 4.8%, p = 0.031). Reference Ren, Xu, Liu, Liu, Wang and Gao23 Mortality rates were also similar to the HERMES collaboration results, ranging from 10% to 20% at 3 months. Reference Campbell, van Zwam and Goyal20–Reference Löwhagen Hendén, Rentzos and Karlsson24 SIESTA had the highest mortality rate at 24.7% in both groups. Reference Schönenberger, Uhlmann and Hacke25 There was a trend toward increased mortality in the CS arm in ANSTROKE, GOLIATH, and the CANVAS pilot; however, these differences were not statistically significant. Reference Simonsen, Yoo and Sørensen21,Reference Sun, Liang and Wu22,Reference Löwhagen Hendén, Rentzos and Karlsson24
Meta-Analyses
Campbell et al., summarized data using meta-analysis from SIESTA, ANSTROKE, GOLIATH, and CANVAS concluding that those managed with GA were more likely to achieve a favorable functional outcome at 3 months (mRS 0–2 GA 49.3%, CS 36.6%, OR 1.71 (CI 1.13–2.59), p = 0.01), a result which may have been partially attributable to higher recanalization rates in those managed with GA (GA 86.2%, CS 74.6%, OR 2.14 (CI 1.26–3.62), p = 0.0050). ICH rates were similar across groups (GA 2.5%, CS 4.9%, OR 0.61 (CI 0.2–1.85), p = 0.38). Reference Campbell, Diprose, Deng and Barber44 Work by Zhang et al., following analysis of results from SIESTA, ANSTROKE, and GOLIATH, also demonstrated improved functional outcomes in those managed with GA (OR 1.87 (CI 1.15–3.03)). Increased successful recanalization (TICI 2b-3) was also seen with GA (OR 1.94 (CI 1.13–3.35)), with no difference in SICH or periprocedural complications. Reference Zhang, Jia, Fang, Ma, Cai and Faramand45
Findings from the anesthesia-specific RCTs and meta-analyses are in contrast to those from the HERMES collaboration. In HERMES, with the exception of MR CLEAN, anesthetic agents and protocols were not prespecified, and as such were more likely to reflect “real-world” practice. In HERMES, those managed without GA had significantly better functional outcomes compared to those managed under GA (covariate adjusted cOR 1.53 (CI 1.14–2.04), p = 0.0044), with 40% in the GA group achieving an independent functional outcome at 3 months compared with 50% in the no GA group (OR 1.65 (CI 1.14–2.38), p = 0.0078). An excellent functional outcome (defined as an mRS 0–1) was also more likely in those managed without GA (GA 23%, no GA 32%, OR 1.68 (CI 1.12–2.52, p = 0.013). These between-group differences were significant in that for every 100 patients managed with GA (rather than no GA), 18 would have poorer functional outcomes and 10 would not achieve functional independence. Reference Campbell, van Zwam and Goyal20
Validity and generalizability of results
Results from nonrandomized and randomized studies evaluating the influence of anesthetic strategy on clinical outcomes in patients with AIS managed with EVT are inconsistent. The outcomes from the nonrandomized studies suffer from selection bias and confounding by indication, but by how much is unknown and whether this is enough to account for differences between these cohort studies and the single center RCTs remains undetermined. There may also be residual confounding due to unmeasured sources of unknown bias. Those with more severe neurologic impairment on presentation (higher NIHSS scores, altered conscious state, agitation) or multiple preexisting comorbidities are more likely to be allocated to GA. This is supported by findings from a post hoc analysis of the International Management of Stroke III trial; patients with a medical indication for GA were less likely to have a good outcome (mRS 0–2 medically indicated GA 19.7%, LA 48%, adRR 0.49 (CI 0.30–0.81), p = 0.005) and had increased mortality (medically indicated GA 33.8%, LA 7.4%, adRR 3.93 (CI 2.18–7.10), p ≤ 0.0001) compared with LA, while there was no difference in probability of good outcome (mRS 0–2 routine GA 40.8%, LA 48%, adRR 0.80 (CI 0.60–1.06), p = 0.12) and mortality (routine GA 13.2%, LA 7.4%, adRR 1.82 (CI 0.87–3.77), p = 0.11) between LA and routine intubation. Higher in-hospital mortality was also seen among the medically indicated GA compared with routine intubation cohort (medically indicated GA 33.8%, routine GA 13.2%, adRR 2.16 (CI 1.09–4.29), p = 0.0274). Reference Abou-Chebl, Yeatts and Yan16
While findings from the anesthesia-specific RCTs suggest that GA is noninferior to CS or LA in patients with AIS undergoing EVT, there are a number of caveats which limit the applicability of these results to other EVT capable centers. Firstly, GA was the standard of care, and CS the intervention, in the majority of the anesthesia-specific RCTs, which may have made it more difficult to demonstrate a benefit of CS over GA. Anesthesia was provided by highly specialized neuroanesthesia and neurocritical care teams able to provide 24 hours in-hospital EVT coverage. Delays due to GA were minimal as teams were fast: arrival to puncture was only 11 minutes in the GA and CS group in the study by Ren et al. Reference Ren, Xu, Liu, Liu, Wang and Gao23 and delays due to GA were 10 minutes or less in SIESTA, GOLIATH, and ANSTROKE, Reference Simonsen, Yoo and Sørensen21,Reference Löwhagen Hendén, Rentzos and Karlsson24,Reference Schönenberger, Uhlmann and Hacke25 in contrast to 20 minutes longer with GA in HERMES (GA 105 minutes (80–149 minutes) vs non-GA 85 minutes (51–118 minutes) p ≤ 0.0001). Reference Campbell, van Zwam and Goyal20 The type and depth of CS varied within and across trials, and a target depth for CS was not provided in some protocols (GOLIATH, ANSTROKE), making comparison across studies difficult. In some cases, the “noGA” group included those managed with both LA and CS (which could be deep), potentially masking a benefit of LA over GA. The small sample size used in each trial further limits the widespread applicability of these results; the largest anesthesia-specific RCT, SIESTA, enrolled only 150 patients. Reference Schönenberger, Uhlmann and Hacke25 Lastly, functional outcomes were only evaluated as a primary outcome measure in ANSTROKE and the study by Ren et al. Reference Ren, Xu, Liu, Liu, Wang and Gao23,Reference Löwhagen Hendén, Rentzos and Karlsson24 Taken together, these limitations suggest that the functionality and expertise of the team in highly experienced centers is a critical factor. Highly experienced, fast teams can use GA effectively and without loss of clinical efficacy of EVT, but this may not be generalizable.
Discussion
Pharmacology and Physiology Considerations
Different anesthetic agents have varying neurochemical, neurophysiologic, and systemic effects. Depending on the type and dose of anesthesia, increases or decreases in cerebral blood flow (CBF) and metabolic demand (CMRO2) can confer both neuroprotective and neurotoxic effects. In general, inhaled or volatile agents (sevoflurane, isoflurane) uncouple CMRO2 from CBF, reducing CMRO2 while increasing CBF in a nonlinear manner; this becomes more apparent with increasing doses. GABAergic drugs (propofol, thiopental) decrease CMRO2 and CBF in a dose-dependent manner. Opioids (fentanyl, sufentanil, remifentanil) result in variable effects on CBF and cerebral metabolic rate which are usually dose dependent and affected by the concomitant use of other anesthetic agents. Reference Sivasankar, Stiefel and Miano46 A reduction in CMRO2 is theoretically beneficial for the ischemic penumbra. Reference Oshima, Karasawa and Satoh47 Inhaled agents have been shown to increase CBF via cerebral vasodilatation, Reference Matta, Heath, Tipping and Summors48 although it has been hypothesized that this may result in an intracranial steal phenomenon resulting in reduced CBF to regions with impaired perfusion. Reference Sivasankar, Stiefel and Miano46,Reference McCulloch, Thompson and Turner49 In contrast, propofol may have vasoconstrictive effects limited to the cerebral circulation reducing CBF. Reference Strebel, Lam, Matta, Mayberg, Aaslid and Newell50,Reference Ravussin, Tempelhoff, Modica and Bayer-Berger51
Inhaled anesthetic agents can be precisely titrated to effect by monitoring the end tidal anesthetic drug concentration. Propofol infusions cannot be monitored in the same way, and if not carefully titrated to clinical signs of anesthetic depth, can result in progressively higher brain and blood concentrations over time, Reference Schüttler and Ihmsen52 potentially with increasing risk for adverse hemodynamic effects. Importantly, ischemic stroke patients are commonly slightly hypovolemic at hospital arrival due to time incapacitated preventing fluid intake, predisposing them to hypotension even with slight sedation-associated increased venous compliance and arterial vasodilation. In the setting of hypotension, volatile anesthesia in particular may result in a substantial reduction in CBF while propofol may have a lesser effect. Reference Van Aken and Van Hemelrijck53
Propofol has been shown to reduce infarct size in animal models, potentially via redistribution of blood flow to the ischemic penumbra (a true Robin Hood syndrome) via cerebral vasoconstriction. Reference Gelb, Bayona, Wilson and Cechetto54 However other studies, including a meta-analysis of experimental stroke in rodents, have demonstrated that while anesthetic agents can reduce neurologic injury by up to 30% (26%–34%, Z = 15, p ≤ 0.001), giving an estimated range of true effects from 3% to 58% (Q = 250, p ≤ 0.001, I 2 = 70%), the neuroprotective effect was not observed in females or in those with comorbidities such as hypertension or diabetes. Reference Archer, Walker, McCann, Moser and Appireddy55 Such findings raise doubt as to whether anesthetic agents could truly exert a neuroprotective effect in the majority of patients presenting with stroke.
The anesthesia-specific RCTs, with the exception of ANSTROKE, utilized the same anesthetic agents in the GA and CS groups to minimize the potential impact of the specific type of drug on outcomes; different doses were used to achieve varying depths of anesthesia. Both SIESTA and GOLIATH reported improved outcomes with propofol GA compared with CS. Reference Simonsen, Yoo and Sørensen21,Reference Schönenberger, Uhlmann and Hacke25 In SIESTA, those managed with propofol GA had improved clinical outcomes (mRS 0–2 GA 37%, CS 18.2%, p = 0.01), Reference Schönenberger, Uhlmann and Hacke25 although absolute rates of functional independence were substantially lower than in comparable trials. Reference Campbell, van Zwam and Goyal20 GOLIATH also reported improved outcomes with propofol GA (mRS 0–2 GA 66.2%, CS 52.4%) despite longer times to recanalization (GA 34 minutes, CS 29 minutes, p = 0.27). Reference Simonsen, Yoo and Sørensen21 As propofol was used for CS in both of these trials, there may have been a beneficial, dose-dependent effect of propofol on outcomes. In ANSTROKE, sevoflurane was used for GA, and mRS at 3 months did not differ between groups (mRS 0–2 GA 42.2%, CS 40%, p = 1.00) Reference Löwhagen Hendén, Rentzos and Karlsson24 suggesting that different anesthetic agents may exert variable effects on outcomes. It may be that, through differing effects on cerebral autoregulation (via effects on CMRO2 and CBF) and secondarily on the ischemic penumbra, the choice and dose of anesthetic agent impact outcomes following EVT.
Cerebral Autoregulation, Blood Pressure, and LVO
Prerecanalization
Under normal conditions, cerebral autoregulation ensures adequate blood flow to the brain despite variations in arterial blood pressure (BP) ranging between 60 and 150 mmHg systolic. Reference Strandgaard56,Reference Paulson, Strandgaard and Edvinsson57 When mean arterial pressure (MAP) is within these limits, changes in arterial tone regulate CBF to meet demand. Reference Jordan and Powers58
Cerebral autoregulation is impaired within hours of acute stroke. Reference Immink, van Montfrans, Stam, Karemaker, Diamant and van Lieshout59–Reference Atkins, Brodie, Rafelt, Panerai and Robinson62 An abrupt reduction in CBF in the setting of an occlusion impairs endothelial cell and receptor function and smooth muscle activation Reference del Zoppo and Hallenbeck63 resulting in maximally dilated arteries (pial collaterals) and arterioles which are unable to adjust their vasoconstrictive response to changes in CBF Reference Jordan and Powers58 ; CBF becomes passively dependent on MAP. Reference Eames, Blake, Dawson, Panerai and Potter64 In the setting of LVO, if MAP is low, as may occur during anesthetic induction, cerebral perfusion pressure falls leading to a reduction in CBF to the penumbra, and potentially extension of the core infarct and a less favorable clinical outcome. The downstream effects of anesthesia-induced hypotension are likely to be more pronounced in patients with baseline hypertension and an altered autoregulatory response (with an upward shift of lower and upper MAP thresholds Reference Strandgaard56 ), particularly in the setting of poor pial collaterals (which are tightly correlated with lower ASPECTs score at baseline) and hyperglycemia on presentation.
During Recanalization/Postrecanalization
BP targets at the time of recanalization and during the early post-recanalization period are not well established. Targets are dependent on a number of factors including baseline ASPECTS, use of thrombolysis or other antithrombotic therapy, timing and extent of recanalization, presence and location of persistent occlusion, presence of mass effect or edema, location of infarction, and age. However, at least theoretically, and in contrast to the prerecanalization period, hypertension is not preferable during this time as it may be associated with an increased risk of reperfusion injury.
Several hemodynamic parameters have been reported to influence clinical outcomes following stroke including a decrease in BP below specific thresholds, Reference Davis, Menon and Baghirzada15,Reference Whalin, Halenda and Haussen65 the extent of BP reduction from baseline, Reference Whalin, Halenda and Haussen65,Reference Löwhagen Hendén, Rentzos and Karlsson66 and BP variability. Reference Jagani, Brinjikji, Rabinstein, Pasternak and Kallmes10,Reference Treurniet, Berkhemer and Immink67 In International Stroke Trial, a higher baseline BP and greater BP variability were both associated with a poorer prognosis. In contrast, an early (within 24 hours) decline in BP (OR 0.93 (CI 0.89–0.97) p = 0.001) and early initiation of antihypertensive therapy (0.78; CI 0.65–0.93; p = 0.007) were associated with improved outcomes, Reference Berge, Cohen and Lindley68 although this may have been influenced by other factors such as baseline stroke severity, early recanalization, and the absence of ipsilateral carotid stenosis.
The anesthesia-specific RCTs targeted a systolic BP (SBP) of 140 mmHg or greater (see Table 4). BP targets were tightly controlled, although hemodynamic changes were still more common in the GA groups in ANSTROKE, GOLIATH, and CANVAS. Reference Simonsen, Yoo and Sørensen21,Reference Sun, Liang and Wu22,Reference Löwhagen Hendén, Rentzos and Karlsson24 In ANSTROKE, MAP was lower with GA (GA 91 mmHg, CS 95 mmHg, p = 0.0484); a fall in MAP greater than 20 mmHg from baseline was also more common (GA 93%, CS 60%, p = 0.003), and more prolonged with GA (GA 22 minutes, CS 15 minutes, p = 0.0432). Those managed with GA required more inotropic support to maintain BP within range (GA 98%, CS 79%, p = 0.0073). Reference Löwhagen Hendén, Rentzos and Karlsson24 CANVAS reported similar results with a lower SBP in the GA group at the time of arterial puncture (GA 125 ± 26 mmHg, CS 159 ± 42 mmHg, p = 0.004) and for 10 minutes afterward (GA 123 ± 21 mmHg, CS 148 ± 33 mmHg, p = 0.007). A decrease in MAP more than 20% baseline was also more common with GA (GA 65%, CS 30%, p = 0.027), although the frequency of MAP decreases more than 40% were similar across groups (GA 15%, CS 10%, p = 1.00). Reference Sun, Liang and Wu22
In GOLIATH, average SBP (GA 143 mmHg, CS 155 mmHg, p ≤ 0.001) and MAP were both lower with GA (GA 95 mmHg, CS 101 mmHg, p ≤ 0.001). SBP (GA 94%, CS 62%, p ≤ 0.001) and MAP were also more frequently below target (GA 91%, CS 46%, p ≤ 0.001), resulting in increased inotrope use in patients managed with GA (phenylephrine and ephedrine, p ≤ 0.001 for both agents). Variations in BP by anesthetic strategy in GOLIATH were not associated with differences in clinical outcome, potentially because neither the amount of time spent with a MAP below target nor MAP at time of reperfusion (GA 97 mmHg, CS 100 mmHg, p = 0.12) differed by anesthetic strategy. Reference Simonsen, Yoo and Sørensen21 In contrast, a post hoc analysis of MR CLEAN reported worse outcomes with a change in MAP in those managed with GA; a MAP 10 mmHg lower than baseline was associated with 1.67 times lower odds of a shift toward a good outcome on the mRS. Reference Treurniet, Berkhemer and Immink67 Whalin et al. also reported a 10% reduction in MAP from baseline as a risk factor for poor outcome (OR 4.38 (CI 1.53–12.56), p = 0.01). Reference Whalin, Halenda and Haussen65 It is possible that more pronounced or prolonged periods of hemodynamic variability occurred in MR CLEAN and the other HERMES trials as highly specified protocols (choice of anesthetic agent, method of administration, depth of sedation, and physiologic targets) were not a key component of the trial design. Aggressive treatment of BP to target, as per the anesthesia-specific RCTs (inotropes were administered to 98% of GA patients in ANSTROKE Reference Löwhagen Hendén, Rentzos and Karlsson24 ), irrespective of the specific type of anesthesia administered, may reduce changes in CBF and its impact on the ischemic penumbra. This in turn may minimize, in part, any deleterious effect of GA on clinical outcomes seen in nonrandomized studies, in centers with ready access to teams able to implement the highly regulated BP protocols of the anesthesia-specific RCTs.
Conclusion
Findings from nonrandomized studies point towards improved functional outcomes without GA in patients with AIS due to LVO of the anterior circulation. This is in contrast to the results from the highly protocol-driven anesthesia-specific RCTs which report improved or no difference in outcomes with GA compared with CS. Strict BP monitoring and treatment to target, with avoidance of severe, prolonged hypotension, alongside fast anesthetic teams with short-time delays likely partially negated any negative impact GA can have on functional outcomes. Interestingly, rates of SICH did not differ by anesthetic strategy in the anesthesia-specific RCTs, weakening the argument that GA is necessary to prevent excessive patient movement to reduce the risk of vessel perforation, a major driver for routine GA use.
A number of outstanding questions remain. What is the optimal anesthetic strategy during EVT? Do different anesthetics and vasopressors with their various effects on brain oxygenation Reference Meng, Cannesson and Alexander69 have variable effects on clinical outcomes? Is their effect modulated by hemodynamic changes and/or collateral status? Do different anesthetics interact with neuroprotective agents such as nerinitide and alter outcomes? Several trials attempting to answer some of these questions are currently in progress including AMETIS, GASS, SEACOAST 1, and CANVAS.
Larger, multicenter RCTs, comparing three different anesthetic strategies (LA, CS, and GA) aimed at primarily assessing the effect on clinical outcomes are required. Ideally these trials will utilize the same anesthetic agent (i.e., propofol for example) across all three groups to minimize further confounding potentially caused by the anesthetic agent itself, standardize the vasopressor of choice to maintain BP within range and take collateral status into consideration. Further work should also consider the potential interaction of anesthesia with neuroprotective agents such as nerinitide. Reference Hill, Goyal and Menon70
Statement of authorship
ELH wrote the draft and revised the manuscript. MDH edited and revised the manuscript.
Conflict of interest
Dr ELH has nothing to disclose.
Dr MDH reports no disclosures related to this review article. Outside of this paper, Dr HILL reports grants from Covidien (Medtronic LLC),; personal fees from Sun Pharma, grants from Boehringer-Ingelheim, grants from Stryker Inc., grants from NoNO Inc., grants from Medtronic LLC, outside the submitted work. In addition, Dr HILL has a patent US Patent office Number: 62/086,077 licensed to Circle Neurovascular Inc., and a patent US Patent office Number: US 10,916,346 licensed to Circle Neurovascular Inc. and owns stock in Pure Web Incorporated, a company that makes, among other products, medical imaging software, is a director of the Canadian Federation of Neurological Sciences, a not-for-profit group, is a director of the Canadian Stroke Consortium, a not-for-profit group, is a director of Circle NeuroVascular Inc., and has received grant support from Alberta Innovates Health Solutions, CIHR, Heart & Stroke Foundation of Canada, National Institutes of Neurological Disorders and Stroke.








