A 59-year-old man presented with a progressive decline in speech. Past medical history was significant for a left middle cerebral artery (MCA) territory stroke 8 years prior to the current presentation (Figure 1A,B,C). Neurologic examination demonstrated global aphasia and preexisting right-sided hemiplegia. Imaging showed a large enhancing lesion within the infarcted area (Figure 1D,E,F). Computed tomography (CT) of the chest, abdomen, and pelvis did not reveal any evidence of other tumours. A presumed diagnosis of a primary neoplastic lesion was made, and the patient underwent craniotomy and subtotal resection of the lesion. There were no intra- or postoperative complications. Final histopathology was consistent with a WHO grade IV glioblastoma. The patient was referred for radiation therapy. His Karnofsky score at 1-month follow-up was 40%.
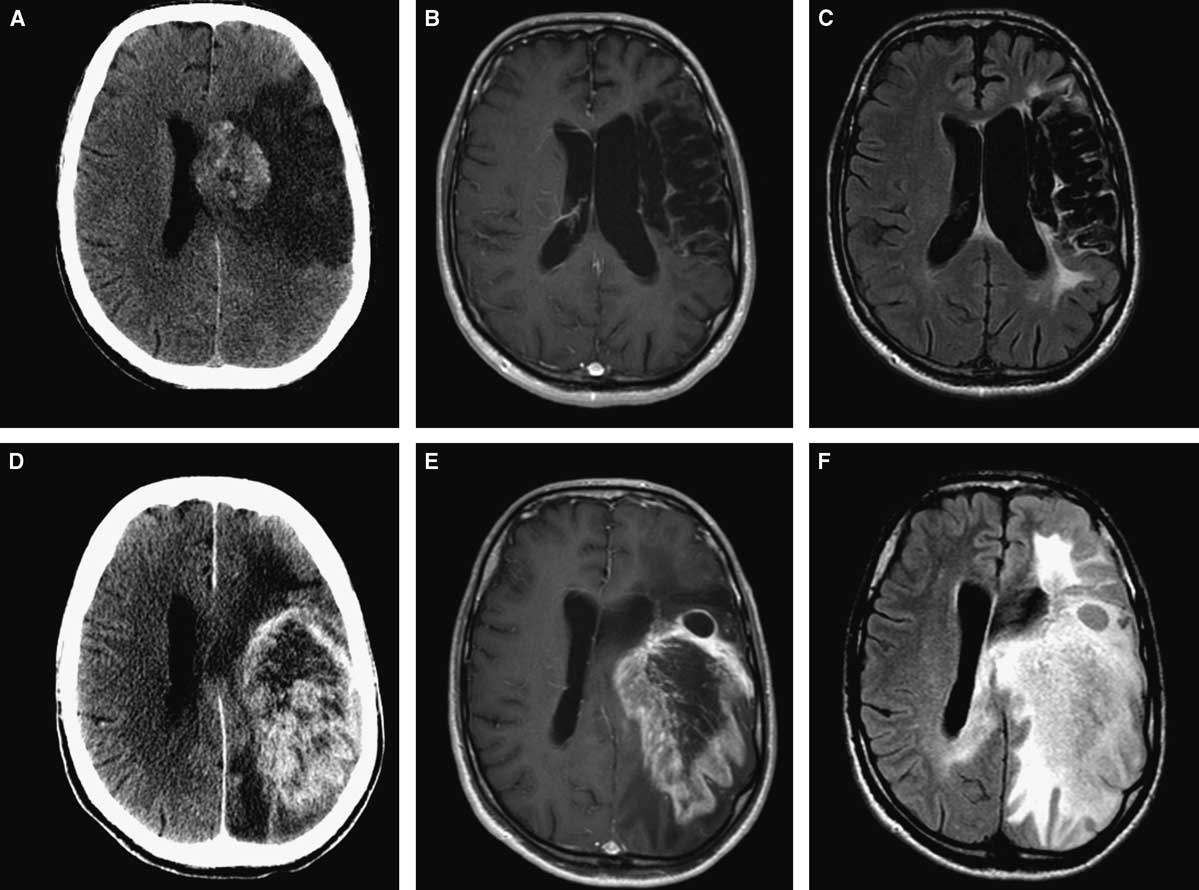
Figure 1 Initial neuroimaging at the time of stroke (A,B,C): axial CT (A), axial MRI with gadolinium (B), and FLAIR sequence (C). Eight years later (D,E,F): axial CT (A), axial MRI with gadolinium (B), and FLAIR sequence (C) showing large intra-axial tumour involving the same area affected by ischemia.
Cases of de-novo glioblastoma have been reported following other intracranial insult or pathology, including penetrating head injury, cerebral contusion, and previous surgical resection.Reference Yaghmour, Kurdi and Baeesa 1 Possible aetiologies that have been proposed include loss of integrity of the blood–brain barrier, the presence of free radicals, and loss of immune surveillance.Reference Kaur, Khwaja, Severson, Matheny, Brat and Van Meir 2 Although there are cases and studies demonstrating the occurrence of infarctions secondary to glioblastoma,Reference Rojas-Marcos, Martin-Duverneuil, Laigle-Donadey, Taillibert and Delattre 3 - Reference Kamiya-Matsuoka, Cachia and Yust-Katz 5 reports of the development of glioblastoma in regions of previous cerebral infarction are exceedingly rare.Reference Ojemakinde, Gonzalez Toledo and Wilson 6 , Reference Wojtasiewicz, Ducruet, Noticewala, Canoll and McKhann 7 Wojtasiewicz et al.Reference Wojtasiewicz, Ducruet, Noticewala, Canoll and McKhann 7 reported the first pathologically proven case of de-novo glioblastoma arising from within a large infracted tissue after 2 years from the time of MCA stroke in a 73-year-old woman. To the best of our knowledge, our case is the only one demonstrating glioblastoma development in an ischemic zone later than 8 years after the time of the ischemic insult.
The present case raises the question of how ischemia and gliosis may play a role in the tumorigenesis of de-novo glioblastoma.Reference Ojemakinde, Gonzalez Toledo and Wilson 6 It has been posited that brain tissue repair mechanisms mirror tumorigenesis and that reactive gliosis may lead to an increased risk of accumulating deleterious mutations.Reference Halliday and Holland 8 Similarly, the relationship between glioblastoma and a hypoxic microenvironment is well-documented, and glioblastomas are distinguished histopathologically from lower-grade infiltrative astrocytomas by characteristic necrotic foci in ischemic zones.Reference Brat, Castellano-Sanchez and Hunter 9 Kim et al.Reference Kim, Fiske and Birsoy 10 recently identified key metabolic pathways that drive survival of these malignant cells in zones of hypoxia. They found high expression of mitochondrial serine hydroxymethyltransferase (SHMT2) in human glioblastoma, which allows cancer cells to reduce their oxygen needs and to be in a metabolic state characterized by prolonged survival in the avascular zones of the tumour.
Taken together, this report raises questions about an important area of glioblastoma biology, demonstrating that this malignant tumour may have a predilection for hypoxic zones of the brain in patients who have suffered ischemic stroke. Future studies aimed at systematically characterizing the epidemiological incidence of glioblastoma post-infarction may be warranted in order to better gauge the clinical impact of this molecularly significant relationship.



