Introduction
Guillain-Barré syndrome (GBS) is a rare but potentially life-threatening disease with an incidence of 1.11 per 100,000 person-years.Reference Sejvar, Baughman, Wise and Morgan 1 Prior infections of various types, be it flu or other infections, have been associated with the development of GBS,Reference Webb, Brain, Wood, Rinaldi and Turner 2 - Reference Cao-Lormeau, Blake and Mons 7 and the clinical disease course and severity of GBS appear to be dependent on the type of preceding infection.Reference Geleijns, Roos and Houwing-Duistermaat 8 - Reference Kuijf, Samsom and van Rijs 11
Effective treatments for GBS involve immunotherapy with intravenous (IV) immunoglobulins (IVIg) and/or plasma exchange (PE).Reference Hughes, Swan, Raphael, Annane, van Koningsveld and van Doorn 12 - Reference Raphael, Chevret, Hughes and Annane 14 For the past decade, the use of IVIg has largely replaced PE as the treatment of choice for GBS due to its treatment effect being similar to that of PE, its convenience in administration, and its good safety profile.
The objective of this retrospective study was to evaluate the effectiveness and safety of Gamunex when administered as the initial treatment of GBS. Our study was a postapproval commitment to Health Canada following licensure of the GBS indication for Gamunex® 10% and IGIVnex® 10% (hereinafter referred to as “Gamunex”).
Methods
Study Design
This study was designed as a retrospective observational study (Figure 1) in which data from patient hospital charts at seven study centers in Canada were collected and reported. Chart review ranged from 2014 to when Gamunex became available in Canada in 2003. The study was designed to collect chart review parameters from 80 consecutive charts of IV Gamunex-treated patients, with at least 20 charts from pediatric patients (<18 years of age) and 20 charts from patients requiring mechanical ventilation within 7 days of admission. The charts were chosen in reverse chronological order at the site level but not across all sites. Of note, the charts for pediatric patients came from two of the seven centers. Some 20 charts of PE-treated patients from this period were to serve as a concurrent control group. Information regarding the demographic characteristics and severity of GBS in both the Gamunex- and PE-treated cohorts were collected.
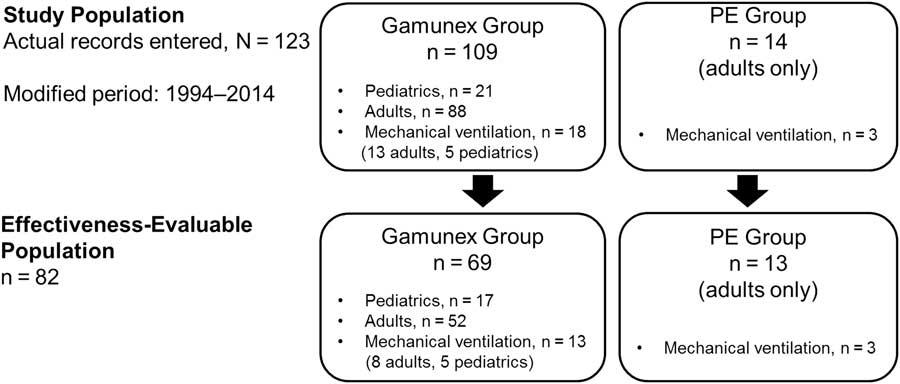
Figure 1 Study diagram.
The study was conducted according to the International Conference on Harmonization Good Clinical Practice guidelines and was approved by the research ethics board of each participating Canadian center.
Inclusion and Exclusion Criteria
Patients were included if they had a documented diagnosis of acute GBS or any variant and had received Gamunex alone or PE alone as therapy within the first 7 days of diagnosis.
Patients were excluded if they had received another IVIg product or PE within 3 months prior to the current GBS admission, had received a second specific GBS therapy <7 days after initiation of their first specific GBS therapy, were enrolled in a GBS interventional clinical trial, or had a change in diagnosis (e.g., chronic inflammatory demyelinating polyneuropathy) at any point during medical care.
Effectiveness Variables
The primary effectiveness endpoint was the proportion of patients with treatment success at either 4 weeks after admission or at hospital discharge, whichever occurred first, compared with the historical success rate for PE treatment as reported in the 2012 Cochrane Review of IVIg versus PE in GBSReference Hughes, Swan and van Doorn 15 and in the 2012 Cochrane Review of PE versus supportive care in GBS.Reference Raphael, Chevret, Hughes and Annane 14 The historical success rate for PE treatment observed in 11 controlled clinical trials 16 - Reference El-Bayoumi, El-Refaey, Abdelkader, El-Assmy, Alwakeel and El-Tahan 26 was 56.70%. To account for heterogeneity across studies, a metaanalysis indicated that the estimated overall success rate of PE was 61.17% (95% confidence interval=49.54% to 72.80%). For the primary effectiveness endpoint in the study, the success rate for Gamunex treatment was set at >55.05% (i.e., 90% of the overall success rate [61.17%] in the published historical PE group).
The denominator for calculating success rate was the number of patients with assessable results of treatment success based on the GBS Disability Scale,Reference Hughes, Newsom-Davis, Perkin and Pierce 27 the abbreviated GBS Disability Scale, or a change in mechanical ventilation status.
The secondary endpoint was the proportion of treatment success in adult Gamunex-treated patients compared to the proportion of treatment success in concurrent PE-treated patients. Other endpoints included the proportion of patients who relapsed and the proportion of patients who were treatment failures. If treatment success, relapse, or treatment failure could not be determined by the available chart data, the case was excluded from the effectiveness-evaluable population. Nevertheless, available safety, demographic, IVIg utilization, and resource utilization data were collected and reported.
Safety Variables
All adverse events (AEs) while receiving Gamunex alone or PE alone and up to 72 hours after the final Gamunex dose or the final PE session were recorded. Any AE occurring more than 72 hours after the final dose where the clinical notes in the patient chart indicated a relationship to the Gamunex or PE treatment were also documented. Adverse events collected from the patient medical charts were classified as adverse reactions (ARs) (defined as any AE occurring during treatment or within 72 hours of the last treatment and any AE occurring more than 72 hours after the last treatment if the medical chart indicated a causal relationship to treatment as unlikely related, possibly related, or related).
Statistical Analysis
Statistical analyses and data presentations were generated using SAS software (v. 9.2 or higher). All statistical inferences were tested two-sided with an α level of 0.05. All data were presented by treatment group (Gamunex or PE). Missing data were identified on the patient chart review form as “not available” or “n/a,” and missing data were not imputed. Treatment success through 56 days was assessed as follows: (1) at least a one-point improvement from baseline on the GBS Disability Scale, (2) at least a one-point improvement from baseline on the abbreviated GBS Disability Scale, or (3) a change in mechanical ventilation status from ventilated at baseline to ventilator-independent around the clock. Relapse was defined as a patient who had deteriorated after an initial improvement and required additional treatment for GBS. Patients who did not meet the aforementioned criteria were classified as failures, as were those who were discharged from the hospital but subsequently readmitted for worsening GBS after initial admission for the identified GBS episode.
Results
A total of 123 hospitalized patients diagnosed with GBS (109 Gamunex-treated and 14 PE-treated) were assessed via retrospective medical chart review (Figure 1). Data from 69 Gamunex-treated cases met the effectiveness-evaluable criteria (52 adults and 17 pediatric patients). Of those 69 patients, 13 (8 adults and 5 children) received mechanical ventilation. Only 4 PE-treated cases were identified from the original time period (2003–2014), and, after extending the date range to 1994, 14 PE-treated cases were identified, of which 13 were evaluable. A total of 3 of the 13 adults in the PE-treated group received mechanical ventilation.
The baseline demographic data are summarized in Table 1. Possible antecedent infection in the 4 weeks prior to the onset of GBS was indicated as a comorbid condition in 79 (72.5%) of the 109 Gamunex-treated patients and in 12 (85.7%) of the 14 adult PE-treated patients. Classic GBS was diagnosed in 72 (66.1%) of the 109 Gamunex-treated patients and in 8 (57.1%) of the 14 PE-treated patients. A total of 21 (17.1%) of the 123 patients in the study received mechanical ventilation and, therefore, were categorized as having severe GBS. Notably, once the diagnosis of GBS was established, Gamunex or PE treatment was generally initiated without delay.
Table 1 Patient hospitalization and baseline demographics

PE=plasma exchange; SD=standard deviation.
Standard dosages and regimens were generally utilized. Overall, the mean total Gamunex dose was 1.94 g/kg (median of 2.00 g/kg) administered over a mean of 3.9 days (median of 5 days). In terms of PE, procedural details were available for 10 patients for whom the majority (n=8) received either 4 or 5–6 PEs. Following completion of acute hospital care for GBS, approximately half of both the Gamunex- and PE-treated patients were discharged to home, and about a third were discharged to a rehabilitation facility.
Effectiveness Data
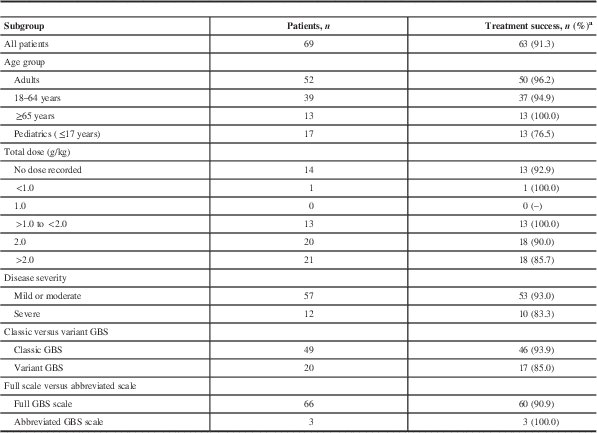
A total of 40 (36.7%) of the 109 Gamunex-treated patients had insufficient data to be evaluated. Taking a conservative approach, if all 40 cases were classified as treatment failures, the success rate of Gamunex was at least 57.8% (63 of 109), which exceeded the prospectively defined effectiveness threshold (Table 2). In the effectiveness-evaluable population, Gamunex treatment was successful in 63 (91.3%) of 69 cases. Of the 14 patients treated with PE, 13 were evaluable, and treatment was successful in 84.6% (11 of 13). Table 3 shows the results for the other endpoints. No cases of GBS relapse were identified in this chart review. Among the 6 Gamunex failure cases, 3 patients (2 adults and 1 pediatric patient) required a second treatment. No PE-treated patients received a second treatment.
Table 2 Primary effectiveness results comparing treatment outcomes of Gamunex-treated patients with historical metaanalysis data of PE-treated patients
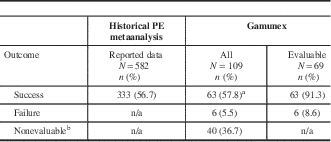
PE=plasma exchange.
Treatment outcomes were assessed based on the Guillain-Barré syndrome (GBS) disability scale. If the GBS Disability Scale was missing, the abbreviated GBS Disability Scale was used.
a A success rate of 55.05% among Gamunex-treated patients (90% of the historical PE success rate of 56.7%) was considered adequate effectiveness.
b Cases were nonevaluable if treatment success, relapse, or treatment failure could not be determined by the available chart data.
Table 3 Treatment outcomes by subgroup of Gamunex-treated patients (effectiveness-evaluable population)

GBS=Guillain-Barré syndrome.
a If the GBS Disability Scale was missing, an abbreviated GBS Disability Scale was used. Subjects without treatment outcome assessments are not included in this table. If the total dose was not specified in the patient chart, it was calculated by adding the individual daily doses (where provided) or multiplying the daily dose by the number of doses.
Safety Data
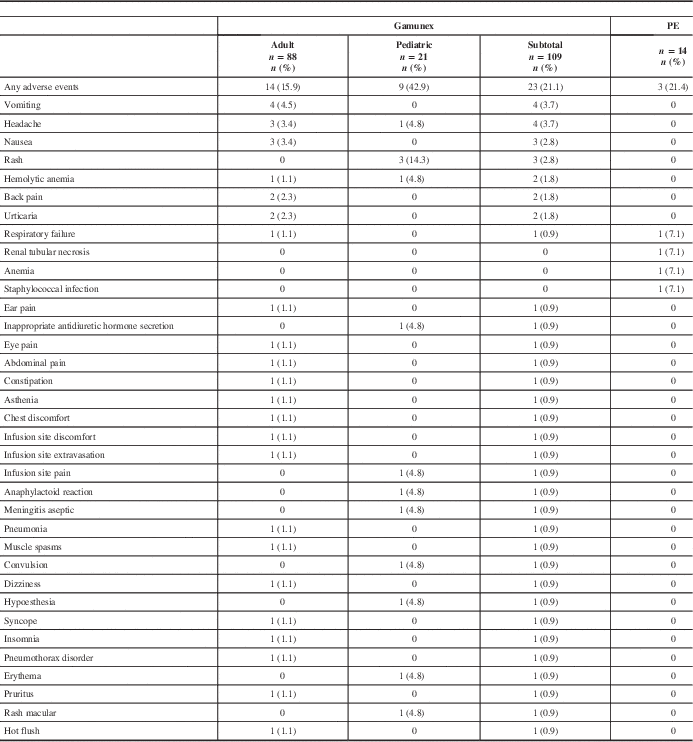
All ARs are shown in Table 4. All recorded AEs met the criteria of an AR, with the exception of two events in the PE group (renal tubular necrosis and anemia) that did not have an onset date recorded, but these events were still reported as ARs. The overall AR rate was similar between treatment groups. Twenty-three (21.1%) of 109 Gamunex-treated patients and 3 (21.4%) of 14 PE-treated patients experienced at least 1 AR.
Table 4 Frequency of adverse events by patient
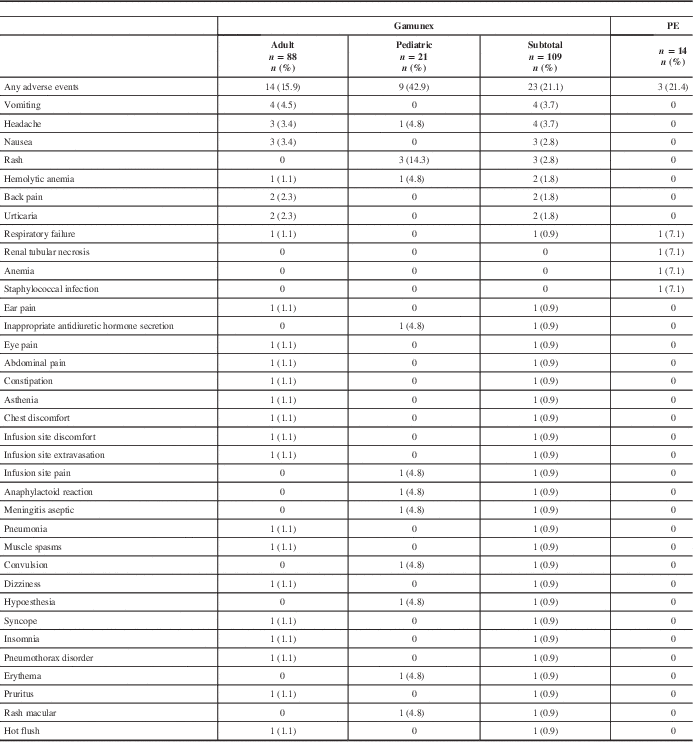
PE=plasma exchange.
Seven serious adverse events (SAEs) occurred among 7 patients (6 Gamunex-treated, 1 PE-treated), of which 2 (both Gamunex-treated) were considered possibly treatment-related. Some 6 Gamunex-treated patients experienced 1 SAE each: pneumonia, pneumothorax, respiratory failure, convulsion, hemolytic anemia, and aseptic meningitis. Two of these—hemolytic anemia and aseptic meningitis in a toddler with concurrent blood culture positive for Acinetobacter—were considered possibly related to treatment. One PE-treated patient experienced one SAE: respiratory failure. Two patients died during the study (one Gamunex- and one PE-treated), both because of respiratory failure.
Interpretation
Despite the limitations inherent in retrospective data extraction from medical charts, the effectiveness of Gamunex demonstrated in this retrospective study was comparable to historical data. Even using a conservative estimate, with all nonevaluable patients (n = 40) classified as treatment failures, Gamunex treatment success was estimated at 57.8% (63 of 109) of patients, exceeding the predefined historical PE effectiveness threshold. When assessing the primary effectiveness endpoint with the evaluable population, the proportion of evaluable Gamunex-treated patients was comparable to the historical effectiveness rate for PE treatment in GBS, with the overall success rate of Gamunex being 91.3% (63 of 69). Secondary endpoints also showed the effectiveness of Gamunex, indicating that this therapy is successful in GBS. Furthermore, the safety profile of Gamunex treatment reported in this study and the AR frequencies were consistent with those reported on Gamunex product labels as well as with the natural clinical course of GBS.
The patients in our retrospective study were demographically similar to GBS patients worldwide. In a recent review,Reference van den Berg, Walgaard, Drenthen, Fokke, Jacobs and van Doorn 28 GBS was reported as more common in males, and the proportion of variant GBS in the current study is comparable to the worldwide experience. A preceding infection in the 4 weeks prior to the onset of GBS was indicated as a comorbid condition in 72.5% of Gamunex- and 85.7% of PE-treated patients, which is consistent with the GBS literature where an antecedent upper respiratory or gastrointestinal infection can be found in up to 90% of affected individuals.Reference Koga, Yuki and Hirata 29 - Reference Willison, Jacobs and van Doorn 31
The choice of GBS treatment is dependent on patient-related factors, socioeconomic considerations, and norms based in part on regional government-based health coverage policies. While PE may appear less costly than IVIg,Reference Winters, Brown, Hazard, Chainani and Andrzejewski 32 , Reference Baranwal, Ravi and Singh 33 the administration of PE requires specialized equipment (often not available in many hospitals), trained personnel, and access to two veins with high flow volumes that may require insertion of a central venous line. PE can also be more difficult to administer in young children due to large volume shifts affecting autonomic cardiovascular stability. IVIg has a response rate that is comparable to that of PE in GBS and requires only one peripheral vein to administer. IVIg does not require any special equipment or training, and it has greater availability than PE in Canada.
Limitations
Comparison of outcomes from current charts with historical published results was impeded by differing standards of care during each time period, different inclusion/exclusion criteria, and different treatment effect assessment scales. While using concurrent PE cases as a control cohort mitigates some of these factors, clinician selection of Gamunex or PE may not be random. Because of the infrequency of use of PE in GBS in recent years, such patients had to be drawn from a time period prior to the availability of Gamunex in Canada (2003), which may introduce a bias due to a period effect. In addition, the subgroup analyses of Gamunex effectiveness are limited by the small numbers of patients in several of the subgroups.
Conclusions
The results from this study show that the success of Gamunex treatment for the treatment of GBS exceeds the predefined historical PE effectiveness threshold at 57.8% (63 of 109 patients) in a conservative estimate, which included all 40 nonevaluable patients classified as treatment failures, and at 91.3% (63 of 69 patients) in the evaluable population. Moreover, the effectiveness of Gamunex (91.3%) was shown to be comparable to PE (84.6%). The risk ratio of Gamunex versus PE was 1.08 (in favor of Gamunex), which was identical to the observed risk ratio from the Cochrane metaanalysisReference Hughes, Swan and van Doorn 15 comparing IVIg to PE, further supporting the effectiveness of IVIg in GBS. The success rates of Gamunex were not notably different among subgroups of patients distinguished by age, total Gamunex dose received, or GBS severity and type. The safety profile of Gamunex in this study was comparable to that reported for Gamunex in the approved product labeling and was consistent with events experienced in the natural clinical course of GBS.
Acknowledgments
The authors wish to thank Dr. Tam Nguyen-Cao of Grifols and Christopher Caiazza for medical writing and editorial assistance.
FUNDING
This study was supported by Grifols, a manufacturer of Gamunex.
DISCLOSURES
KC, KH, and EM are employees of Grifols, a manufacturer of Gamunex. VB has consulted for Grifols, CSL Behring, BioNevia, Lilly, Pfizer, Dainippon, Sumitomo, and Eisai, and has received research grant support from all of these. ZAS has received unrestricted educational grants and research funding from CSL Behring and Grifols.
Statement of Authorship
Each author was a significant contributor to the writing, critical review, and editing of the manuscript from outline through to the final version. All authors approved the final manuscript. ZAS and VB were investigators who participated in the study and have been involved in clinical interpretation of the findings. KC, KH, and EM are Grifols employees and were instrumental in the study design, conduct, analysis, and interpretation of study results as the program director and study manager, medical monitor, and clinical development head, respectively.