Status epilepticus is “a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms that lead to abnormally prolonged seizures”.Reference Trinka, Cock and Hesdorffer 1 Epilepsia partialis continua (EPC) is a rare form of focal status epilepticus defined by clonic muscular twitching affecting a limited part of the body occurring for a minimum of 1 hour, and recurring at intervals of no more than 10 seconds.Reference Bien and Elger 2 The diagnosis requires the presence of epileptiform electroencephalographic discharges or other neurophysiological abnormalities demonstrating the cortical origin of the muscle jerks.Reference Bien and Elger 2
Here we describe the clinical and electrophysiological features of an elderly man with EPC of the abdominal musculature following an acute ischemic stroke.
A 91-year-old man was admitted to our hospital with an acute right-sided hemiplegia and somnolence, which had appeared the day before. On admission, myoclonic jerks of the right hemibody were noticed, which promptly stopped after levetiracetam (1000 mg intravenous) administration. His medical history was notable for severe Alzheimer's disease (diagnosed 22 years earlier), chronic vascular encephalopathy with parkinsonism, moderate renal failure, and pancreatic carcinoma. The head CT scan showed ischemic lesions in the left hemisphere, more prominent in the fronto-parietal and temporal lobe, with marked chronic vascular encephalopathy and leukoaraiosis (Figures 1 and 2); the radiological features of the left temporal hypodensity were suggestive of a subacute cerebral infarction. The day after the admission, continuous involuntary contractions of the trunk were noted. Neurological examination showed continuous involuntary and rhythmic jerks involving the abdominal muscles, not spreading to other parts of the body (video). The electroencephalogram (EEG) (Figure 3) revealed rhythmic epileptiform discharges of triphasic/diphasic morphology admixed with polyspikes confined to the left hemisphere (Supplementary material). The most prominent epileptiform abnormalities were localized, over the left posterior temporo-parieto-occipital area. Equipotentiality of epileptiform discharges occurred between electrodes P3 and O1, sometimes with maximal amplitude over O1>P3≥T5>T3, C3 (more evident in referential montage). There were also independent epileptiform discharges located anteriorly, close to the anterior Sylvian region in the left fronto-temporal area, sometimes showing phase reversal at F7 or equipotentiality between F7 and T3. A periodicity (frequency of about 1 Hz) was sometimes evident in epileptiform discharges occurring in both posterior and anterior regions (i.e., lateralized periodic discharges). The EEG recording also showed brief ictal transitions seen maximally over the left posterior hemispheric structures, especially around T5. Overall, the EEG showed anterior and posterior areas of epileptogenicity, more marked in the left posterior temporo-parieto-occipital region. The myoclonic movements were not synchronous with the rhythmic epileptiform discharges. A concomitant electromyographic recording of the muscles of the abdominal wall showed bursts of quasirhythmic asymmetric polymorphic motor potentials of the right abdominal recti muscles, with a duration lower than 100 ms and a frequency of 0.5-1 Hz. The movements attenuated after intravenous administration of lorazepam (4 mg) and levetiracetam (2000 mg), but then reappeared. The patient died of cardiac arrest the following day.
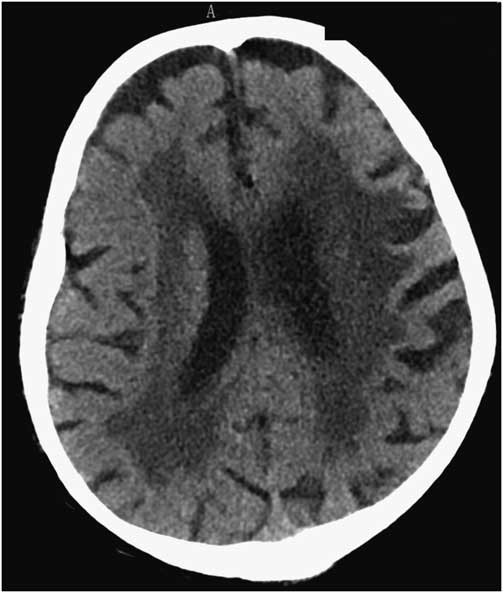
Figure 1 Head CT scan showing marked chronic vascular encephalopathy and leukoaraiosis.
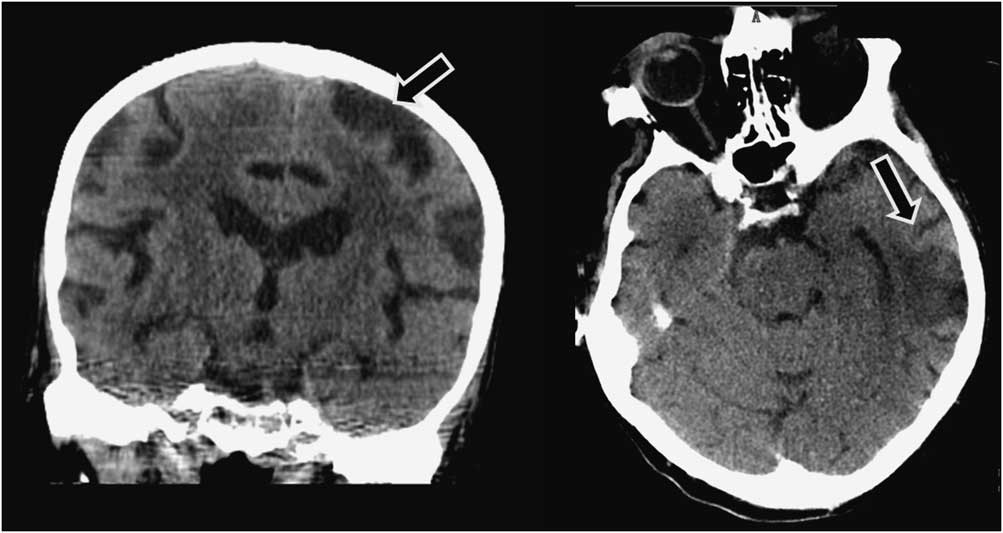
Figure 2 Left: head CT (coronal reconstruction) scan showing a left fronto-parietal ischemic lesion (arrow). Right: head CT (axial) scan showing a left temporal ischemic lesion (arrow); the radiological features of this temporal lesion are suggestive of a subacute infarction.
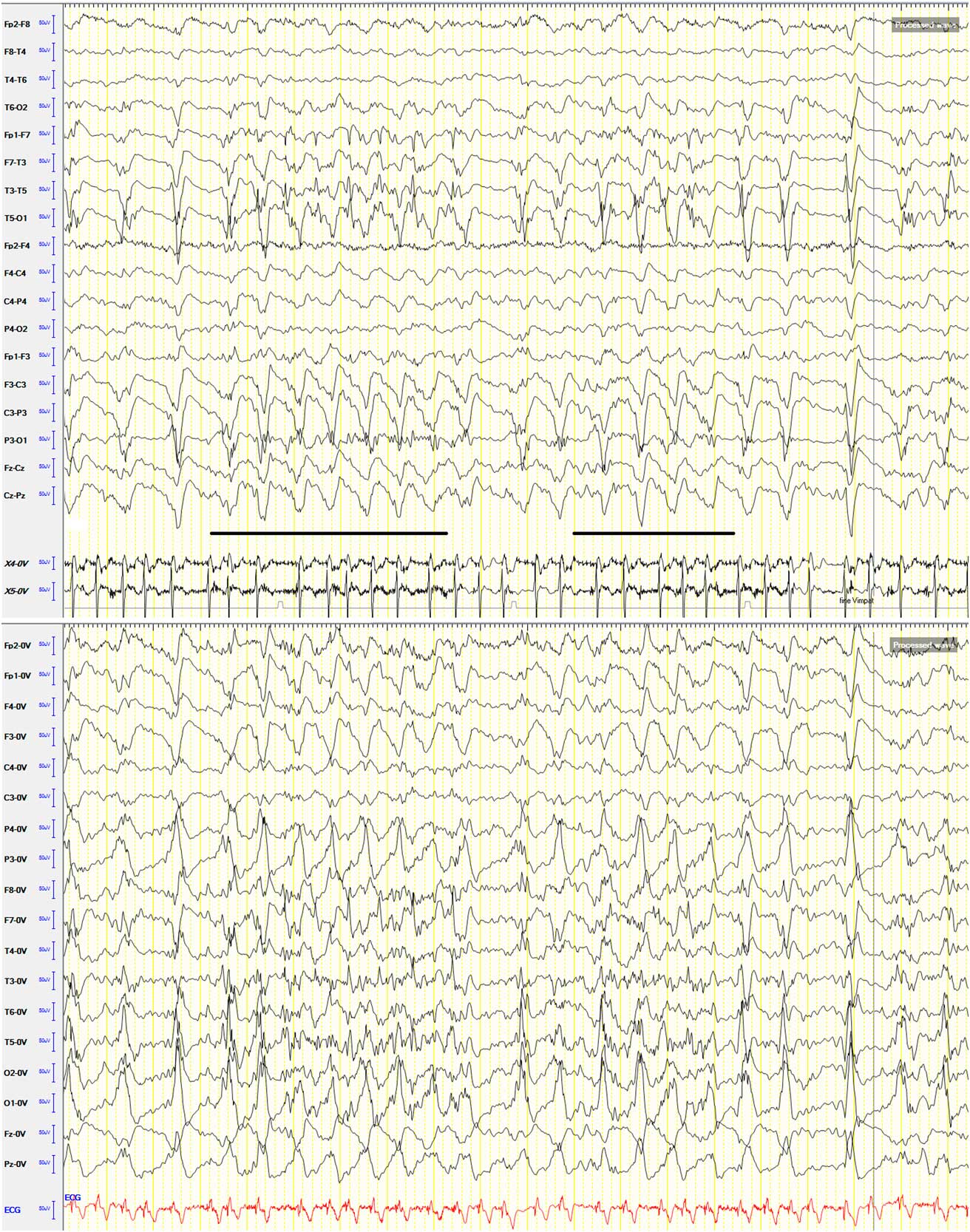
Figure 3 EEG shown in the longitudinal bipolar and referential (common average reference) (below) montage; sensitivity: 7 μV; low-frequency filter: 0.53 Hz; high-frequency filter: 70 Hz. Speed: 20 seconds/page. EMG recording with surface electrodes from both abdominal recti muscles: X4-0V: left rectus abdominis muscle; X5-0V: right rectus abdominis muscle. The most prominent epileptiform abnormalities are localized over the left posterior temporo-parieto-occipital area. Equipotentiality of epileptiform discharges can be seen between electrodes P3 and O1, sometimes with maximal amplitude over O1>P3≥T5>T3, C3 (more evident in referential montage). There are also independent epileptiform discharges located anteriorly, close to the anterior Sylvian region in the left fronto-temporal area, sometimes showing phase reversal at F7 or equipotentiality between F7 and T3. A periodicity (frequency of about 1 Hz) can be observed in epileptiform discharges occurring in both posterior and anterior regions. There are also brief ictal transitions seen maximally over the left posterior hemispheric structures (underlined in the longitudinal bipolar montage), especially around T5.
The presence of continuous involuntary movements with electromyography (EMG) correlates of twitches lasting <100 ms (even if not time-locked to the rhythmic epileptiform discharges), the epileptiform discharges in the EEG, and the attenuation of the myoclonic jerks after anti-epileptic therapy confirmed the epileptic nature of the symptoms,Reference Obeso, Rothwell and Marsden 3 leading to the diagnosis of EPC. These electroclinical features rule out alternative diagnoses, including a propriospinal myoclonus.
The anatomical localization of the epileptogenic zone underlying EPC of the abdominal muscles or trunk is thought to be in the frontal parasagittal or parietal cortex around the central lobe.Reference Ribeiro, Sousa, Teotónio, Bento and Sales 4 Involvement of abdominal musculature in focal motor seizures is rare; this may be explained by the small representation area of the abdomen on the motor cortical homunculus.Reference Tezer, Celebi, Ozgen and Saygi 5
Only two previous cases of EPC of the abdominal musculature due to ischemic stroke have been published;Reference Ribeiro, Sousa, Teotónio, Bento and Sales 4 unlike other etiologies, in both cases post-stroke EPC was preceded by focal myoclonic seizures on the hemibody contralateral to the side of the vascular event. However, unlike our case, they did not occur in the context of acute cerebrovascular lesions.
As our case shows, focal-onset motor seizures and even EPC may selectively involve the abdominal musculature.
It is noteworthy that in our patient the most prominent epileptiform abnormalities were recorded over the left posterior temporo-parieto-occipital area, whereas minor abnormalities were seen more anteriorly, in the left fronto-temporal region. The periodicity of epileptiform discharges occurring in both posterior and anterior regions, together with the lack of evolution in discharge amplitude, frequency, morphology, and distribution, is consistent with lateralized periodic discharges in the setting of an acute ischemic stroke. Of note, the myoclonic movements observed in this patient were not synchronous with the rhythmic epileptiform discharges. A strict neurophysiological association between this epileptiform activity and the myoclonic jerks is therefore difficult to prove with certainty. At least 10 cm2 of synchronous cortical activity is required to record an ictal rhythm on scalp EEG,Reference Tao, Ray, Hawes-Ebersole and Ebersole 6 and this explains why in more than 50% of EPC cases the scalp EEG is completely normal.Reference Bien and Elger 2 Furthermore, this dissociation between epileptiform discharges over the motor cortex and the motor correlates can be also owing to vertical inhibition.Reference Elger and Speckmann 7 In this phenomenon, spiking in lamina V following local penicillin administration leads to contralateral twitching without concomitant epileptiform activity recorded on the cortical surface. A motor correlate of epileptic activity was observed only when epileptic discharges involved the superficial and deep cortical layers.Reference Elger and Speckmann 8 The EEG recording also showed brief ictal transitions seen maximally over the left posterior hemispheric structures, especially around T5 (Figure 3), suggesting that these graphoelements might represent the EEG correlate of symptoms. However, unlike the abdominal jerks, these elements lasted only a few seconds, making a definite temporal and topographic relationship between the EEG activities and myoclonus difficult to demonstrate. Overall, it is therefore possible that in our patient the myoclonic jerks of the abdominal musculature actually had no (or very minimal) EEG correlate, and that the lateralized periodic discharges resulted from the acute cerebral infarct (and the concomitant severe underlying leukoaraiosis) but were unrelated to clinical symptoms. If so, the absence of a straight correlation between myoclonic movements and epileptiform discharges prevents a precise anatomical localization of the epileptogenic zone underlying EPC.
Epilepsia partialis continua of the abdominal musculature can have different etiologies,Reference Trinka, Cock and Hesdorffer 1 , Reference Obeso, Rothwell and Marsden 3 , Reference Ribeiro, Sousa, Teotónio, Bento and Sales 4 , Reference Chalk, McManis and Cascino 9 , Reference Aljaafari, Nascimento, Abraham, Andrade and Wennberg 10 and is a phenomenon that has been reported only rarely. Previous reported cases of EPC with involvement of the abdominal muscles have suggested that this phenomenon may be because of epileptogenic zones not restricted to the somatotopic representation of abdominal musculature (e.g., parasagittal regions, parietal, or frontal lobe),Reference Ribeiro, Sousa, Teotónio, Bento and Sales 4 , Reference Aljaafari, Nascimento, Abraham, Andrade and Wennberg 10 owing to complex organization of the homunculus and some individual variabilityReference Tezer, Celebi, Ozgen and Saygi 5 . A post-stroke EPC of the abdominal muscles with an epileptic focus in the occipital lobe has also been reported, with the following pathophysiological mechanisms proposed to explain the final activation of the truncal area in the motor cortex from seizures originating posteriorly: (1) spread of seizure activity from occipital (supracalcarine) regions to frontal and parietal regions, and (2) functional reorganization of the cortical tissue adjacent to a brain injury (neuroplasticity) with altered activation patterns.Reference Ribeiro, Sousa, Teotónio, Bento and Sales 4 The latter mechanism is more likely to occur in cases with a long time interval between the brain lesion, such as the stroke and the onset of epileptic seizures, as neuroplasticity takes time to develop.Reference Ribeiro, Sousa, Teotónio, Bento and Sales 4 In our case, EPC manifested <48 hours after the onset of stroke symptoms; alterations in cortical networks owing to neuroplasticity as a mechanism underlying EPC are therefore not plausible. The spread of epileptic activity from posterior to anterior regions, giving rise to motor phenomena, is also difficult to prove, as the EEG did not clearly reveal a propagation of ictal activity. Indeed, if symptoms were due to spread of epileptic activity, other areas of the primary motor cortex with seizure threshold lower than that of the abdominal area would have been activated, leading to other symptoms, including hemiclonus. However, the fact that the patient first presented with hemiclonus points to an epileptogenic zone clearly located in the contralateral primary motor cortex. It is therefore possible that, following the acute brain injury, the cortical hyperexcitability initially involving a larger cortical region subsequently restricted to a more discrete area of the homunculus.Reference Penfield and Jasper 11 In this case, symptoms could have been the result of direct ictal generation in the truncal area rather than a cortical activation secondary to propagation from a nearby region.Reference Aljaafari, Nascimento, Abraham, Andrade and Wennberg 10 This necessarily implies a very focal epileptic discharge arising within a small cortical area, which could have no clear EEG ictal correlates.
Subcortical regions may also have played a role in the pathogenesis of EPC in this patient, considering the entity of the chronic vascular leukoencephalopathy and leukoaraiosis. Similarly, it is also possible that the coexistent Alzheimer's disease may have contributed in altering the cortical excitability. However, owing to lack of available data, this remains speculative.
In conclusion, a combined EEG–EMG recording in the event of involuntary continuous muscular jerks in the abdominal musculature should be performed to reach a diagnosis. An ischemic stroke in the middle cerebral artery territory can alter the excitability of the primary motor cortex, lowering the seizure threshold in the trunk motor area, where threshold is postulated to be high,Reference Oster, Aljumairi and Cosgrove 12 leading to EPC or motor seizures involving the abdominal muscles.
Statement of Authorship
FB, VT, AB and RN contributed to data collection and analysis. FB and RN contributed to writing/editing of this article.
CONFLICT OF INTERESTS
This study was not funded. FB has received speakers' honoraria from Eisai and PeerVoice, payment for consultancy from Eisai, and travel support from Eisai, ITALFARMACO, and UCB Pharma. Other authors have no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.330





