Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is one of the most common forms of hydrocephalus observed in adults. It equally affects men and women and the average age of onset seen so far is around 70 years.Reference Williams and Malm1 It is usually characterized by the Adams triad of disturbance of gait, cognitive impairment, and urinary incontinence. Gait disturbance is the most pertinent and predominant symptom present in the majority of the patient population. Cognitive impairments occur later in the disease.Reference Williams and Relkin2 The most effective treatment plan in iNPH is the cerebrospinal fluid (CSF) tap.Reference Toma, Papadopoulos, Stapleton, Kitchen and Watkins3 Around 60%–80% of patients improve following CSF tap in these patients [1]. Although the clinical importance for this disease is known, the pathophysiology of gait impairment remains unclear.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4
Previous studies have demonstrated that impaired functional connectivity between the prefrontal regions, the basal ganglia, and the motor cortex is responsible for abnormal gait in iNPH.Reference Momjian, Owler, Czosnyka, Czosnyka, Pena and Pickard5-Reference Sasaki, Ishii and Kono7 Transcranial magnetic stimulation (TMS) has been used as a tool to assess the corticospinal motor pathways in the pathophysiology of gait disturbance in patients with iNPH.Reference Zaaroor, Bleich, Chistyakov, Pratt and Feinsod8 Short-interval intracortical inhibition (SICI) has also been found to be abnormal in them.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4
In the present study, we aimed to study the cortical inhibition and facilitation properties of the brain in patients with iNPH that may help in understanding the pathophysiology of gait impairment in these patients.
Methods
The study was conducted in the Department of Neurology at the National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, India. The study was approved by the Institute Ethics Committee and written informed consent was obtained from all the participants. Our study included 11 patients with iNPH (probable NPH) and 13 healthy controls (HCs). The diagnostic criteria proposed by Relkin et al. was used to select the patients.Reference Relkin, Marmarou, Klinge, Bergsneider and Black9 All the patients with iNPH had symptoms of gait abnormality, urinary disturbance, cognitive impairment, and evidence of NPH on MRI brain.Reference Relkin, Marmarou, Klinge, Bergsneider and Black9 Patients with a history of epilepsy, presence of cardiac pacemakers, cochlear implants, and any other metallic implants in the body were excluded. Patients with a chronic medical illness such as cardiovascular, renal and hepatic diseases, and diabetes mellitus were also not considered for the study. The severity of parkinsonism was assessed using the unified Parkinson’s disease rating scale (UPDRS) motor part III in ON and OFF state for all the patients. In addition, the iNPH grading scale (iNPHGS) and the gait scale were used to assess the severity of gait dysfunction. The iNPHGS assesses cognition impairment and urinary disturbance in addition to gait disturbance. The gait scale consists of walking score, step score, and time score. Cognitive performance of all the participants was assessed using mini-mental status examination (MMSE), Montreal cognitive assessment (MoCA), and Addenbrooke’s cognitive evaluation III (ACE-III).Reference Folstein, Folstein and McHugh10-Reference Mathuranath, Cherian, Mathew, George, Alexander and Sarma12 Subjects underwent the handedness criteria as per Edinburgh’s handedness inventory.Reference Oldfield13
TMS Methodology
TMS was done using Magstim 200 stimulator with a hand-held figure-of-eight coil. Standard procedure and precautions were followed for all measurements based on the recommendations of the IFCN committee.Reference Rossini, Burke and Chen14 TMS was performed in the sitting position while subjects relaxed in a comfortable chair. The optimal scalp position (“hot spot”) for the left motor cortex for hand and leg area was identified separately and marked. The area was then stimulated to elicit motor responses in the contralateral first dorsal interosseous (FDI) muscle in the hand and tibialis anterior (TA) in the leg. Two Ag–AgCl electrodes were used to record the surface muscle response. For recording motor evoked potentials (MEPs) in the upper limb, the electrodes were placed in a belly-tendon montage with the active electrode placed over the belly of the right FDI and the reference electrode was placed on the metacarpophalangeal joint of the right index finger. For recording MEPs in the lower limb, the electrodes were placed on the right TA muscle in a similar belly-tendon montage. The surface muscle responses were monitored to ensure lack of significant muscle activity. The coil was positioned over the motor area of the hand such that the handle was pointing backwards at an angle of 45° to the sagittal plane to stimulate the FDI, and for the leg motor area stimulation, the coil was placed horizontally over the vertex with the handle pointing backwards at 180° to the sagittal plane.
The stimulus intensity was increased gradually in 5% increments until a satisfactory MEP was obtained. This was repeated for 10 consecutive trials and the responses recorded and saved. For this study, TMS parameters such as the resting motor threshold (RMT), central motor conduction time (CMCT), contralateral and ipsilateral silent periods (cSPs and iSPs), short-interval intracortical inhibition (SICI), and intracortical facilitation (ICF) were recorded in the upper limb and RMT and CMCT in the lower limb.
RMT (expressed as %) was defined as the minimal stimulus intensity required to evoke a MEP of at least 50μV from peak-to-peak in a relaxed muscle in 50% of 10 consecutive trials. RMT was acquired for both the upper and lower limb. CMCT was determined by eliciting the MEP using stimulus intensity of 120% of RMT. Spinal stimulation was done above the C7 vertebral spinous process for recording MEP in the upper limb and over the lower thoracic spinous processes for the recording of MEP in the lower limb. CMCT was then calculated as the difference between the latencies of MEP obtained by cortical and spinal stimulation. CMCT was expressed in milliseconds. SP was recorded using suprathreshold stimulus intensity applied during voluntary contraction of the FDI. Both cSP and iSP were measured in 5 out of 10 consecutive trials. SICI was recorded using subthreshold conditioning stimulus that was 80% of the RMT and test stimulus of 120% of RMT separated by an interstimulus interval (ISI) of 2 msec and ICF was recorded with ISI of 10 msec under the same stimulation condition.
Cognitive Assessment
Neuropsychological assessment was done using a combination of three dementia screening questionnaires, i.e., MMSE, MOCA, and ACE-III, Indian version in all the subjects. iNPH patients were assessed at baseline and within 24 h of drainage lumbar puncture (LP) to note any difference in scores immediately following CSF drainage. The scores were compared between them to record the pattern of cognitive impairment between the groups.
Lumbar Drainage Procedure
Patients with iNPH underwent lumbar drainage with the removal of 30–40 ml of CSF. The procedure was done under aseptic precautions with the patient lying in the lateral position. Routine cytology and biochemical analysis of the CSF was performed. Post procedure, the clinical assessment (iNPHGS and gait scale) was done at 6, 12, and 24 h to look for improvement in the symptoms. Those patients who improved after the CSF drainage underwent repeat TMS and cognitive evaluation using the same protocol 24 h of the procedure.
Statistical Analysis
The statistical analysis was performed using R software.Reference Bathke, Harrar and Madden15 The normality of data was checked using the Shapiro–Wilk test. For comparison of mean values among patients and controls, the Mann–Whitney U test was used. Spearman’s correlations were performed to check the relationship between clinical, cognitive variables, and TMS measures. A Bonferroni corrected value of <0.05 was taken as statistically significant.
Results
Demography
Eleven patients with iNPH (2 females and 9 males) and 13 HCs (7 females and 6 males) were included in the study. The mean age of the patients was 69.00 ± 6.71 years for iNPH and 54.38 ± 4.43 years for controls. The mean scores of MOCA, MMSE, ACE-III, and UPDRS III score (OFF and ON) between iNPH and controls were recorded and shown in Table 1. In the pre-LP iNPH group, there was no significant difference in the UPDRS-III ON and OFF score (p = 0.23). Six patients were on L-dopa therapy but none of them had any response to therapy.
Table 1. Demographic and clinical details of patients with iNPH and HC
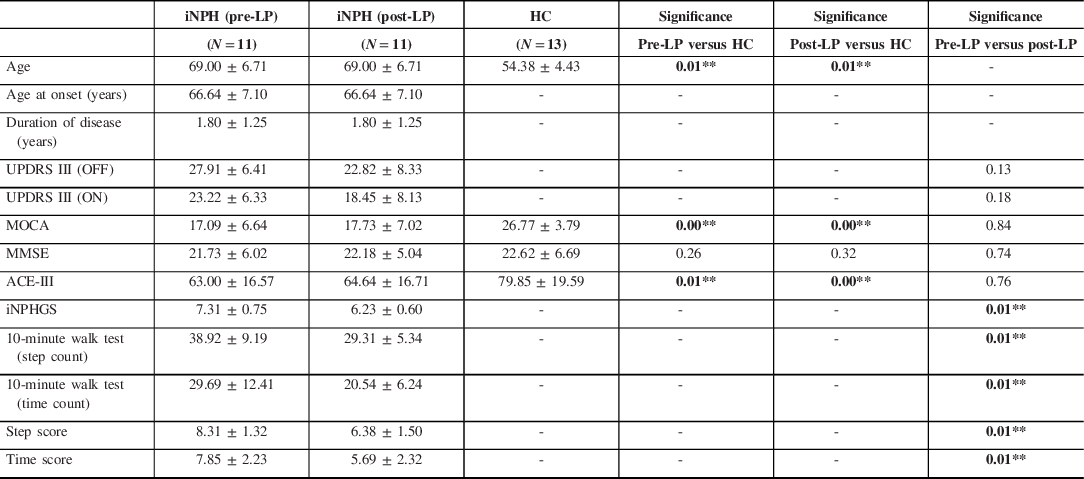
ACE-III = Addenbrooke’s cognitive evaluation III; HC = healthy control; iNPH = idiopathic normal pressure hydrocephalus; iNPHGS = idiopathic normal pressure hydrocephalus grading scale; MMSE = mini-mental status examination; MOCA = Montreal cognitive assessment; Post-LP = post-lumbar puncture; Pre-LP = prior to lumbar puncture; UPDRS III = Unified Parkinson’s disease rating scale.
* p < 0.05, ** p < 0.01.
Transcranial Magnetic Stimulation
Comparison of Pre-LP iNPH and HC
There were significant differences found only in the CMCT for the lower limb (CMCT-LL) when compared with controls (22.43 ± 7.21 vs, 14.59 ± 5.66, p < 0.01) (Table 2). We also found significant differences in the iSP between patients and controls (56.43 ± 20.02 vs, 29.63 ± 14.14, p < 0.01) and SICI was decreased significantly as compared to controls (1.26 ± 1.10 vs, 0.40 ± 0.53, p < 0.01).
Table 2. Comparison of TMS parameters of patients of iNPH with HC
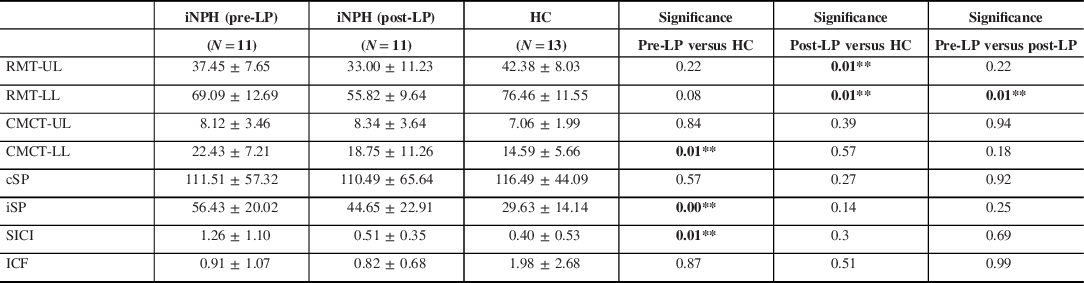
CMCT-LL = central motor conduction time for the lower limb; CMCT-UL = central motor conduction time for the upper limb; cSP = contralateral silent period; HC = healthy control; ICF = intracortical facilitation; iNPH = idiopathic normal pressure hydrocephalus; iSP = ipsilateral silent period; Pre-LP = prior to lumbar puncture; Post-LP = post-lumbar puncture; RMT-LL = resting motor threshold for the lower limb; RMT-UL = resting motor threshold for the upper limb; SICI = short latency intracortical inhibition; TMS = transcranial magnetic stimulation.
* p < 0.05, ** p < 0.01.
Comparison of Pre- and Post-LP iNPH Patients
There was a significant improvement in the RMT of lower limb stimulation (p = 0.012) (Figure 1). There was also a reduction in the lower limb CMCT after LP (from 22.43 ± 7.21 to 18.75 ± 11.26), but it was not significant (p = 0.19). Also, the SICI normalized after LP, but the result was not significant (p = 0.69) (Figure 2).
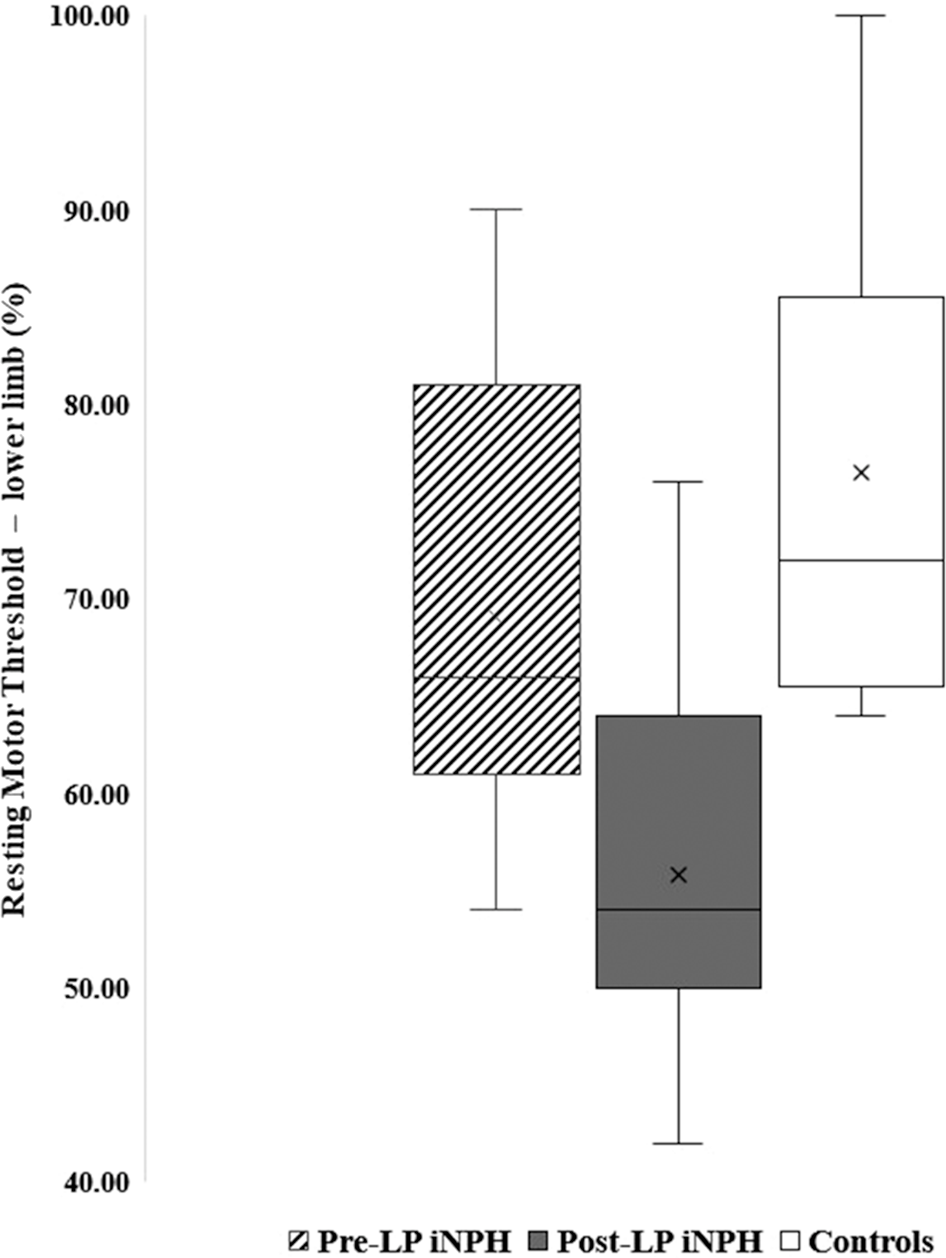
Figure 1. Box plot showing the RMT of lower limb in pre- and post-LP NPH patients and controls.
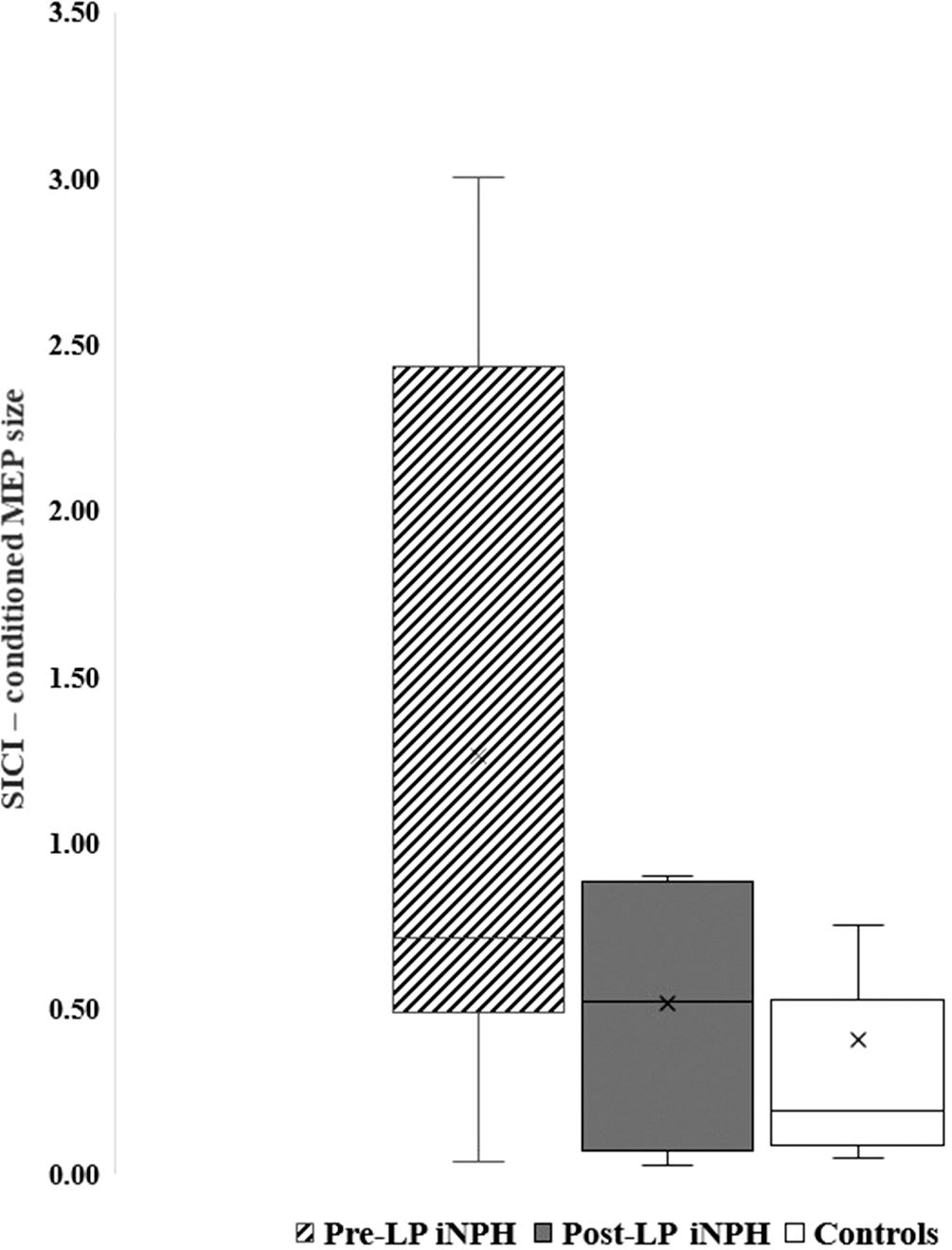
Figure 2. Box plot showing the SICI in pre- and post-LP NPH patients and controls.
Comparison of Post LP NPH and HC
There was a significant difference in the lower limb RMT between the controls and the post-LP iNPH patients (69.09 ± 12.69 vs. 55.82 ± 9.64, p < 0.01) (Figure 1). There was a mild prolongation of the lower limb CMCT in patients with iNPH (18.75 ± 11.26) when compared with HC (14.59 ± 5.66) but was not statistically significant (p = 0.57). SICI regained (0.51 ± 0.35 vs. 0.40 ± 0.53, p = 0.30), however, was not significant (Figure 2).
Cognitive Evaluations
Comparison of pre LP NPH and HC
The mean MoCA, MMSE, and ACE III scores were 17.09 ± 6.64, 21.73 ± 6.02, and 63.00 ± 16.57, respectively, in the iNPH patients (Table 1). Similarly, the mean MoCA, MMSE, and ACE III scores were 26.77 ± 3.79, 22.62 ± 6.69, and 79.85 ± 19.59, respectively, in HC. There was a significant difference observed in the MoCA between iNPH and HC (p = < 0.01) and in ACE-III scores (p = < 0.01).
Comparison of Pre and Post LP iNPH
There was no change in the cognitive scores (MMSE, MoCA, and ACE III) between the two groups.
Comparison between Post LP iNPH and HC
The mean MoCA, MMSE, and ACE III scores were 17.73 ± 7.02, 22.18 ± 5.04, and 64.64 ± 16.71, respectively, in the iNPH patients. Similarly, the mean MoCA, MMSE, and ACE III scores were 26.77 ± 3.79, 22.62 ± 6.69, and 79.85 ± 19.59, respectively, in HC. There was a significant difference observed in the MoCA between iNPH and HC (p = < 0.01) and in ACE-III scores (p = < 0.01).
Correlations
Correlations for the pre-LP group revealed that MOCA was negatively correlated with the RMT for the upper limb (RMT-UL) (r = −0.65, p < 0.05) and the CMCT-LL parameter (r = −0.68, p < 0.05). The mean MMSE scores also negatively correlated with the RMT-UL parameter (r = −0.62, p < 0.05). The RMT for the lower limb (RMT-LL) positively correlated with the age of onset (r = 0.73, p < 0.01) and negatively correlated with the duration of disease (r = −0.83, p < 0.01).
Correlations for the post-LP group found significant correlations between RMT-LL and age (r = 0.60, p < 0.05), age at onset (r = 0.79, p < 0.01), and duration of disease (r = −0.85, p < 0.01). The CMCT-LL was significantly correlating with age of the patients (r = 0.64, p < 0.05). The cSP was significantly correlating with the age (r = 0.74, p < 0.01) and age at onset of the patients (r = 0.65, p < 0.05).
Discussion
In our study, we evaluated RMT, CMCT of both the upper and lower limbs, iSP and cSP for upper limbs using single pulse, and SICI and ICF of upper limbs using the paired-pulse protocol. In our study, the baseline RMT-UL and RMT-LL were less when compared to HC, which reduced further following LP drainage. In addition, the CMCT-LL also reduced following LP. There was a minimal reduction in the cSP and iSP following LP. There were an abnormally reduced SICI found in the pre-LP stage. Enhancement in SICI was noted after LP but did not reach significance and there was no change in the ICF. Our study demonstrates that the RMT-LL was significantly reduced in the patients with iNPH post LP. The gait score improved significantly along with the step score and time score. There were no significant differences in cognitive parameters post LP. The mean scores of MOCA and ACE-III were significantly different when compared to controls.
Whether gait dysfunction is a result of an imbalance in neurotransmitter systems localized to the cortex with resultant changes in excitability in NPH and vascular parkinsonism has long been a matter of debate. TMS with its various single- and paired-pulse protocols and even triple stimulation technique seems to be an ideal method to answer these questions. The RMT-LL parameter was significantly low in iNPH pre and post which continues to be the most prominent feature in the disease suggesting that there might be a reduced skull-to-cortex distance due to ventricular enlargement.Reference Kozel, Nahas and deBrux16 Furthermore, the low RMT-LL can be attributed to the hyperexcitability of the motor cortex due to weak inhibition from subcortical regions.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4 RMT values at baseline were similar to controls, and the lower limb and upper limb values decreased further after LP, contrary to observations made by Chistyakov et al.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4 Their study postulated that restoration of cortical GABA receptor-mediated inhibition as the most likely explanation for their results, which were obtained 1 month post successful shunt surgery, where the RMT values progressed toward higher values of HCs. We postulate that there might be a transient increase in cortical excitability post LP, mediated by sodium channels, in cortical or sub-cortical motor networks which tends to normalize over a while, especially after successful shunt placement, largely due to enhanced GABAergic transmission.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4 This needs to be confirmed with studies having a large sample size or a meta-analysis of a few such studies as ours. This large difference or a shift from sodium channels to GABA-mediated output is difficult to explain and requires further exploration by a serial prospective study in a large number of patients, inducted immediately following surgery. SPs, both cSP and iSP, were unaffected in both of these groups which are in agreement with all previous studies. SP reflects GABAB activity and is probably unaltered in iNPH. In a recent study with TMS, Raffaele Nardone et al. (2019) studied cholinergic transmission with short latency afferent inhibition and found that its reduction in iNPH patients correlated with gait and memory problems. They also confirmed the presence of reduced RMT and SICI in pre-surgical iNPH patients as seen in our patients.Reference Nardone, Golaszewski and Schwenker17
Although not significant, SICI enhanced post LP which can be attributed to the dysfunction of ƴ-aminobutyric acid-A which plays a key role in mediating the effects of frontal lobe dysfunction on motor tasks. This study demonstrates disinhibition as a possible mechanism of gait dysfunction which is normalized following successful shunt placement. SICI is thought to mediate by GABA neurotransmitter system and its decrease is a non-specific finding in many neurologic conditions. However, its restoration following shunt placement, correlating with improvement in gait dysfunction, seems to be a promising marker in predicting post-surgical outcomes, if demonstrated in the immediate post CSF tap test when gait improvement is most likely to occur, i.e., first 24 h.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4
ICF, on the other hand, which represents cortical glutamatergic activity, is influenced by both GABAA agonist and NMDA antagonist.Reference Ziemann, Lönnecker, Steinhoff and Paulus18 ICF in our study was decreased post LP within 24 h of the procedure. Hence, we postulate that reduced facilitation in our study, post LP could be due to restoration of GABAergic activity. This is similar to the study by Chistyakov et al., where they found restoration of inhibition in patients 1 month following shunt placement with significant changes in SICI.Reference Chistyakov, Hafner, Sinai, Kaplan and Zaaroor4
In our study, there were significant differences in the CMCT-LL between pre-LP iNPH patients and controls; however, a non-significant reduction in CMCT-LL was observed between pre- and post-LP patients. This may be attributed to the TMS done after 24 h in comparison to Chistyakov et al. who performed the test after 1 month of shunt placement. In patients with NPH, due to the ventricular dilatation that may cause stretching of the lower limb corticospinal fibers causing impairment of conduction (Yakovlev’s hypothesis).Reference Yakovlev19 This may suggest that there may be involvement of the lower limb corticospinal tracts due to the ventricular dilatation and can be useful in pre-surgical prediction. As our sample size was small, large studies can provide a better picture. In a study by Zaaroor et al., patients with prolonged CMCT did not improve following shunt surgery, while those patients with normal CMCT did improve.Reference Zaaroor, Bleich, Chistyakov, Pratt and Feinsod8 This is contrary to our observations. This study does not support the theory of pyramidal tract involvement as a cause of gait impairment. Hence, it is likely that in addition to the impaired conduction in the stretched corticospinal fibers, there may be other unknown mechanisms that cause prolonged CMCT, gait impairment, and subsequent improvement after lumbar drainage.
In our study, the MMSE and MoCA negatively correlated with the RMT-UL. This suggests that an increase in the RMT, i.e. reduced cortical excitability, is probably associated with cognitive impairment. This may be a unique feature in patients with iNPH as patients with Alzheimer’s dementia have reduced RMT.Reference Khedr, Ahmed, Darwish and Ali20,Reference Chandra, Issac, Nagaraju and Philip21
The fact that our study had a small sample size and TMS was done within 24 h of LP, even though successful, could be the most plausible explanation for such a dichotomy. Studies with large sample size and follow-up starting immediately after shunt placement and continuing for a long period can help us understand this paradox. In our study, we did not include patients who did not improve after CSF drainage, which could have provided predictive information in relation to intervention.
Conclusions
Patients with pre-LP iNPH had a significant prolongation of the lower limb CMCT when compared to controls. In addition, these patients had significant prolongation of iSP and reduced SICI with no difference in the RMT-LL. Lumbar CSF drainage in them resulted in a significant reduction in lower limb RMT when compared to both the controls and pre-LP status, thereby suggesting an increase in cortical excitability. Post LP, there was an enhancement of SICI. These results support the view that impaired control of the motor output due to altered connectivity in the motor network is related to gait disturbances in iNPH. In addition, a non-significant reduction in the CMCT-LL following lumbar drainage may suggest an improvement in corticospinal conduction. This indicates that TMS can be a very useful tool in evaluating lower body parkinsonism. However, larger studies are warranted to validate our findings.
Conflict of Interest
The authors have no conflicts of interest to declare.
Statement of Authorship
PKP: conception of the study, study design, supervision, data review, and manuscript review. AA: recruitment of study participants, administration of TMS, cognitive assessment, data collection, and manuscript writing. AB: administration of TMS, statistical analysis, data collection, and manuscript writing. NK: recruitment of study participants, administration of TMS, and manuscript review. RY: study design, supervision, data review, and manuscript review.






