Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease affecting the central nervous system that predominantly attacks myelin in the brain and spinal cord.Reference Chiaravalloti and DeLuca1 About 40 to 65% of patients with MS have cognitive deficits.Reference Chiaravalloti and DeLuca1 These cognitive deficits are associated with poor functional status,Reference Staples and Lincoln2 as well as decreased quality of lifeReference Cutajar, Ferriani and Scandellari3 and productivity.Reference Campbell, Rashid and Cercignani4,Reference Rao, Leo, Ellington, Nauertz, Bernardin and Unverzagt5 The functions predominantly affected in MS are episodic memory,Reference Brassington and Marsh6,Reference DeLuca, Barbieri-Berger and Johnson7 working memory,Reference Grafman, Rao, Bernardin and Leo8 information processing speed,Reference DeLuca, Chelune, Tulsky, Lengenfelder and Chiaravalloti9–Reference Denney, Sworowski and Lynch12 attention,Reference Paul, Beatty, Schneider, Blanco and Hames13 executive functionsReference Foong, Rozewicz and Quaghebeur14 and visuospatial functions.Reference Vleugels, Lafosse and Nunen15
Although many patients are aware of their cognitive deficits and report these difficulties to their health professionals, patients’ assessments of their own cognitive status are not always accurate.Reference Goverover, Kalmar and Gaudino-Goering16–Reference Demers, Rouleau, Scherzer, Ouellet, Jobin and Duquette20 Whereas the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) is used frequently, the results obtained by the self-reported measures (MSNQ-P [patient form]) do not always accurately reflect objective cognitive functioning in patients with MS.Reference Goverover, Kalmar and Gaudino-Goering16,Reference Benedict, Cox, Thompson, Foley, Weinstock-Guttman and Munschauer21,Reference Dagenais, Rouleau and Demers22 It is, therefore, possible that a patient without any subjective complaint on the MSNQ-P would nonetheless present clinically significant objective cognitive deficits on a more in-depth assessment. Unfortunately, in the absence of subjective complaints on the MSNQ-P, many patients with cognitive deficits thus fail to receive appropriate referral for comprehensive neuropsychological evaluation. This has important clinical implications given the known impact of cognitive dysfunctions on personal and professional life.Reference Staples and Lincoln2–Reference Rao, Leo, Ellington, Nauertz, Bernardin and Unverzagt5
Over the years, a number of neuropsychological test batteries have been developed specifically to evaluate patients with MS’ cognitive abilities by assessing the functions that are preferentially affected in MS.Reference Korakas and Tsolaki23 Many of these tests, such as the Brief Repeatable Battery of Neuropsychological Tests (BRBN)Reference Rao24 and the Minimal Assessment of Cognitive Function in MS (MACFIMS),Reference Benedict, Cookfair and Gavett25 show good sensitivity but are too time-consuming for more widespread administration.
Because it is neither realistic nor appropriate to perform an exhaustive neuropsychological evaluation of all patients with MS, clinicians need a short, sensitive and reliable screening test that could rapidly detect the presence of cognitive impairment and lead to referral for a more complete neuropsychological evaluation. To reduce testing time, researchers have developed the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS),Reference Langdon, Amato and Boringa26 a short version of the MACFIMS. While the validity of the BICAMS has been demonstrated, the lack of executive function assessment has been criticisedReference Gromisch, Zemon and Holtzer27 since executive deficits have been reported in at least half of patients with MS regardless of their level of cognitive impairment.Reference Migliore, Ghazaryan and Simonelli28 To compensate for these limitations, a shortened version of the MACFIMS battery was studied by Gromisch and his team.Reference Gromisch, Portnoy and Foley29 In this version, called aMACFIMS, only some trials of the original tests are administered. It achieved higher specificity but lower sensitivity than the BICAMS. However, the tests which are shortened lose their psychometric properties and, especially for memory tests (CVLT-II and BVMT-R), can no longer be used with the tested patients because of familiarity with the material (e.g. same words, same geometric figures) and practice effects.
Many clinicians and researchers use the Symbol Digit Modalities Test (SDMT)Reference Smith30 as a screening test. Its quick administration time (5 minutes) and its high sensitivity to the cognitive deficits experienced by patients with MSReference Kalb, Beier and Benedict31 explain its widespread use. However, it only assesses the speed of information processing, which is often but not always affected in MS. Some use the Mini Mental State Examination (MMSE),Reference Folstein, Folstein and McHugh32 but it does not include items to assess executive and attentional functions, which makes it less appropriate as a screening tool for MS.Reference Beatty and Goodkin33,Reference Scherer34
In contrast, the MoCA test is particularly adapted to cognitive screeningReference Nasreddine, Phillips and Bedirian35 of patients with MS since it evaluates many of the cognitive functions known to be preferentially affected in MS.Reference Dagenais, Rouleau and Demers22,Reference Amato, Zipolo and Portaccio36 In addition, the MoCA test is very accessible since it is free of charge and available in more than 30 languages. Since September 2019, a certification is mandatory to administer the MoCA, except for students, residents, fellows and neuropsychologists. Administration time is less than 10 minutes, an advantage for patients with MS, who often report fatigue. Studies have already shown that MoCA scores are significantly lower in patients with MS compared to healthy controlsReference Abraham and Rege37,Reference Aksoy, Timer, Mumcu, Akgün, Kivrak and Rken38 and that the test is sensitive to the type of cognitive impairment noted in MS.Reference Dagenais, Rouleau and Demers22 However, no study to date has investigated the use of the MoCA in patients with MS without subjective complaints.
The aim of this study was to fill this gap in the literature by assessing the efficacity of the MoCA test in detecting the presence of subtle objective cognitive deficits among patients without subjective complaints, given the fact that a significant proportion of these patients do show cognitive deficits upon objective testing. To achieve this aim, patients without subjective cognitive impairment – as reported by the MSNQ-P – were screened for cognitive impairment with the MoCA and their scores were compared with their results on the MACFIMS neuropsychological test – the gold standard for this study. We hypothesised that the MoCA test score would be a valid screening tool to discriminate cognitively impaired from cognitively intact patients and indicate who should be referred for detailed neuropsychological evaluation.
Methods
Participants
This study used data from a previous investigationReference Rouleau, Roger, Langlois, Nadeau and Duquette39 on the effect of the beta-interferon medication Rebif® on clinical evolution (work status and quality of life) in treated vs. untreated patients with MS. In that study, 111 patients treated with Rebif exclusively for at least 2 years and up to 18 years were compared to 185 patients, matched in age, gender, education level, age at disease onset and disease duration, who never received disease-modifying drugs. Among the 296 patients with MS, a subgroup of 121 patients (52 treated and 69 untreated) agreed to complete the MACFIMS. Since there was no effect of treatment group on cognitive functions observed, the two groups were combined for the present study. All participants were recruited from the MS Clinic of the Centre Hospitalier de l’Université de Montréal (CHUM). The CHUM ethics committee approved this study and every participant signed an informed consent form.
Among the initial sample of 121 patients who completed the MACFIMS, only those without subjective cognitive complaints were selected, that is, those who scored below 24 on the MSNQ-P,Reference Benedict, Cox, Thompson, Foley, Weinstock-Guttman and Munschauer21,Reference O’Brien, Gaudino-Goering, Shawaryn, Komaroff, Moore and DeLuca40 leaving a final sample of 98 patients (19 men and 79 women) with MS. To be included in the project, patients had to meet the following criteria: (1) diagnosed with MS (clinically isolated syndrome, relapsing–remitting or secondary progressive) according to the 2005 Revision of the McDonald Diagnosis Criteria (Polman et al., 2005); (2) followed at the CHUM’s MS Clinic within the last 2 years; (3) aged 18 years or over; (4) EDSS ≤ 5.5; (5) able to read and write in French. Patients were excluded from the study if they met any of the following criteria: (1) had a history of drug abuse, neurological or developmental disorders, or psychiatric or other medical conditions that could affect their neuropsychological performance (e.g. traumatic brain injury, stroke); (2) were unwilling or unable to consent; (3) or were diagnosed with primary progressive MS.
Measures
Screening Tests
The MoCA and the MSNQ were used as screening tests for cognitive impairment.Reference Korakas and Tsolaki23,Reference Rao24 In addition to the total score (/30), the MoCA includes the following sub-scores: (1) visuospatial and executive functioning, (2) naming, (3) attention (e.g. simple attention, working memory, vigilance), (4) language (e.g. repetition, phonemic fluency), (5) abstraction, (6) delayed free recall and (7) orientation. The MSNQ was completed by the patient (MSNQ-P) and a close relative (MSNQ-I). The MSNQ-P score was used to confirm the absence of subjective cognitive complaint by the participants. A score under 24 met this criterion.Reference Benedict, Cox, Thompson, Foley, Weinstock-Guttman and Munschauer21,Reference O’Brien, Gaudino-Goering, Shawaryn, Komaroff, Moore and DeLuca40
Exhaustive Neuropsychological Testing
Exhaustive neuropsychological assessment was conducted using the MACFIMS. Normative data available for each test (see below) were used to compare our sample to healthy controls. Impairment on each measure was defined as a cut-off z score of −1.5. The presence of objective cognitive impairment was defined as failure of two or more tests on the MACFIMS battery.
Verbal fluency was evaluated with the French version of the Controlled Oral Word Association Test (COWAT) with the letters P–F–L.Reference Benton, Hamsher and Sivan41 This task measures oral production of words beginning with a specific letter in a limited period of time, excluding proper nouns, numbers and the same word with a different suffix.Reference Lezak, Howieson and Loring42 Normative data of French-speaking Quebec adults adjusted for age and education were used for this version of the COWAT.Reference St-Hilaire, Hudon and Vallet43 Visuospatial functioning was assessed by the Judgment of Line Orientation Test (JLO),Reference Benton, Hamsher, Varney and Spreen44 which evaluates the ability to visually match 30 pairs of angled lines. Original norms of the JLO were used.Reference Benton, Hamsher, Varney and Spreen44 Information processing speed was measured using the SDMTReference Smith45 and the Paced Auditory Serial Addition Test (PASAT-3).Reference Gronwall46 For the SDMT, participants must orally pair specific numbers with given geometric symbols as quickly and accurately as possible. On the PASAT-3, participants must add 60 pairs of randomised digits by adding each new digit to the one heard immediately prior to it. Normative data from a study by CentofantiReference Centofanti47 were used for SDMT, and norms from RaoReference Rao, Leo, Bernardin and Unverzagt48 were utilized for PASAT-3. Executive functions were evaluated by the Sorting Test, a subtest of the D-KEFS, and normative data from the D-KEFS examiners’ manual were used.Reference Delis, Kaplan and Kramer49 In this subtest, participants are asked to sort cards that share either perceptual or verbal features to form and explain as many categories as possible. The Brief Visual Memory Test (BVMT-R) was used as a measure of visuospatial memory and original norms were used for scoring.Reference Benedict50 In this task, participants have 10 seconds to observe six geometric stimuli presented visually, and then must draw as many stimuli as they remember in the correct location. Verbal memory was assessed by the California Verbal Learning Test (Second Edition) (CVLT-II) and normative data from the CVLT manual were used.Reference Delis, Kramer, Kaplan and Ober51 This task evaluates recall and recognition of verbal material using a 5-trial presentation of a 16-word list (list A) and a single presentation of an interference list (list B). At each trial, examiners read the entire list and participants are asked to recall as many words as possible.
Procedures
All evaluations took place at the CHUM and participants were recruited at the MS clinic at a follow-up visit with their neurologist. If the patient met the criteria for inclusion, informed written consent was obtained, and the patient was scheduled for a neuropsychological evaluation.
A neuropsychology graduate student, under the supervision of a certified neuropsychologist, performed the evaluation. First, the patient was questioned about the psychosocial background, including education, family status and occupation/employment. They were then given the MSNQ-P to complete. Following administration of the neuropsychological test battery, the patient was given a number of questionnaires to complete at home, including the Multiple Sclerosis Quality of Life (MSQOL)-54, which contains questions pertaining to mood (emotional well-being scale), pain and fatigue (energy scale) and the MSNQ-I to be given to a relative. The examination also included an assessment of the level of the patient’s disability according to the Expanded Disability Status Scale (EDSS).Reference Kurtzke52
Statistical Analysis
To make a direct comparison between MoCA test scores and performance on the MACFIMS possible, a global MACFIMS score was calculated by averaging each standardised z score obtained for the various tests included in the MACFIMS. Direct comparisons between patients with MS who are cognitively intact and those who are cognitively impaired (2 or more tests < −1.5 SD on the MACFIMS battery)Reference Kim, Zemon and Rath53 were performed by computing t tests on total MoCA test scores and on the results obtained on the different sections of the MoCA test. A hierarchical multiple regression analysis was carried out with the global score on MACFIMS as the dependent variable. Demographic, MSQOL variables and MoCA subtests were added gradually in three blocks as independent variables. This allowed us to examine what percentage of variance in the MACFIMS global score could be explained by these variables.
Finally, to validate the clinical use of MoCA as a screening test, ROC curves were generated for the total MoCA score and scores obtained on its different subsections to determine the best cut-off to separate cognitively impaired from cognitively intact patients.
Results
There was no statistical difference between patients with MS who completed the MACFIMS (N = 121) and those who did not (N = 175) in terms of age (t [294] = 1.07, p = .089), education (χ2 [3, N = 296] = 6.61, p =.086), gender (χ2 [1, N = 296] = 0.044, p = .834), duration of the disease (t [275] = 1.31, p = .190), MS course (χ2 [2, N = 296] = 0.513, p =.774) and EDSS score (t [294] = 0.902, p = .368).
Among the 121 patients who completed the MACFIMS, 98 did score below 24 on the MSNQ-P, confirming the absence of cognitive complaints in this patient group. This final sample included 19 men and 79 women, aged 26 to 71 years (Mean = 49.57; SD = 11.40) who had completed between 8 and 18 years of education (Mean = 14.56; SD = 2.77). Duration of the disease ranged from 4 months to 35 years (Mean (years) = 10.75; SD = 7.61). Data on the sociodemographic situation, patient status and duration of illness are presented in Table 1. Despite the absence of cognitive complaints according to the MSNQ-P, 23 out of the 98 patients were classified as cognitively impaired (23.5% of the sample), having failed at least 2 tests on the MACFIMS battery. Table 2 shows the frequency of failures for each of the MACFIMS subtests that support this classification. It is worth mentioning that only 10.2% (10/98) of the sample was found to be impaired on SDMT while a higher proportion of patients were impaired on COWAT, BVMTR and CVLT-II, which suggests that some patients are intact on the SDMT although they have impaired memory and executive functions. Indeed, in our sample, among the 23 patients classified as impaired on the MACFIMS (2 or more tests < -1.5 SD), only 8 (34.8%) were impaired on the SDMT. The results of the cognitive screening tests are presented in Table 3.
Table 1: Patients’ sociodemographic profile and disease characteristics
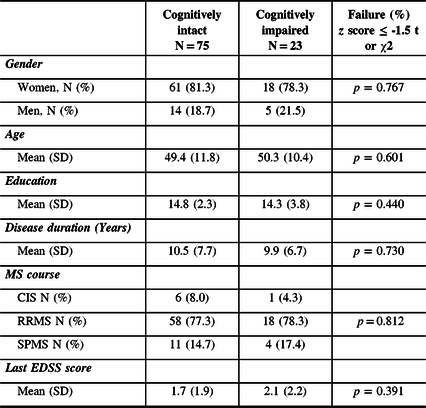
Table 2: Performance on the MACFIMS: frequency of failures (%)
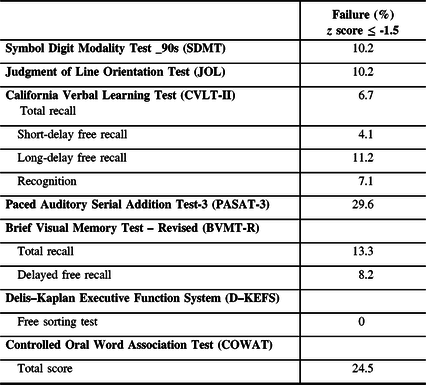
Table 3: Results of cognitive testing

Relationship Between the MSNQ-P and MACFIMS Scores
There was no significant correlation between the MSNQ-P score and the result of the neuropsychological evaluation including the MoCA test (total score and sub-sections), the scores obtained on all the tests on the MACFIMS battery, or the global score on the MACFIMS.
However, the MSNQ-I was significantly correlated with the MoCA total score (r = -0.246, p = 0.017) and the global score on the MACFIMS (r = -0.278, p = 0.007). As shown in Table 3, no difference was found between cognitively impaired and cognitively intact patients on the MSNQ-P (t[96] =-0.297, p = 0.767), whereas these two subgroups were statistically different on the MSNQ-I (t[92] = -3.720, p <.001).
Relationship Between the MoCA and MACFIMS Scores
As shown in Table 3, a t test reveals that patients who were classified as cognitively impaired had a lower MoCA score than those who were considered cognitively intact (t[96] = 5.6, p <.001). In addition, the results obtained in three sub-sections of the MoCA: visuospatial/executive (t[96] = 5.61, p < .001), verbal fluency (χ2 [1, N = 98] = 12.94, p < .001) and delayed recall (t [98] = 3.35, p = .001) were significantly different between the two groups, whereas scores obtained in naming, attention, orientation, abstraction and sentence repetition were not statistically different.
In light of these results, hierarchical multiple regression analyses were performed with the global score on MACFIMS as the dependent variable. A summary of the results is shown in Table 4. Age, duration of illness, EDSS score, sex and education did not significantly explain the variance of the global score on MACFIMS (p =.191). The inclusion of depression and fatigue also did not explain an additional portion of the variance (p =.102). MoCA test scores significantly explain 42.6% of the variance in the global score on MACFIMS. Examination of the regression coefficients (see Table 5) shows that only the visuospatial/executive score, verbal fluency and delayed free recall were significantly related to the global score on MACFIMS (visuospatial executive: β = .228, p = 0.021; verbal fluency: β = .406, p <.001; delayed free recall: β = .297, p = 0.002), and not the orientation and attention scores.
Table 4: Summary of hierarchical regression analyses

***p < .001.
Table 5: Regression coefficients of each predictor of the global score on MACFIMS

*p < .05; ** p < .01 ***p < .001.
Sensitivity and Specificity of the MoCA Test
With a cut-off score of 27, ROC curve analysis (AUC = 0.815, 95% CI, .714 -.916, p < .001) yielded a sensitivity of 87% and a specificity of 68% for the total score on the MoCA test. The best sensitivity/specificity ratio was obtained with the complete MoCA test (Youden index [YJ]Reference Youden54 = 0.550). The three sub-scores that were significant in the regression analysis (executive/visuospatial, verbal fluency, delayed recall) demonstrated a potential value for classifying patients, but their Youden indices were not as high as that achieved with the complete MoCA scale (Figure 1).

Figure 1: Results of the ROC curve for the Montreal Cognitive Assessment in patients with multiple sclerosis without cognitive complaints
Discussion
The important contribution of this study is that it assesses cognitive impairment in patients without subjective cognitive complaints. Despite the absence of cognitive complaints as assessed by the MSNQ-P, the results of this study demonstrate the relevance of performing objective cognitive screening tests in patients with MS with the MoCA, especially in light of the known impact of cognitive deficits on professional and personal life. Although the informant version of the MSNQ (MSNQ-I) appears to be more accurate than the patient version (MSNQ-P) in assessing the presence of cognitive deficits, it is not as precise as objective testing and is often not available in a clinical context. Indeed, many patients, particularly those with a low level of disability (low EDSS score), do not come to a medical appointment accompanied by a person who knows them well enough to give an accurate account of their daily functioning and cognitive status as required by the MSNQ-I.
Our study found evidence of subtle cognitive deficits in patients with no subjective complaints and demonstrated the validity of the MoCA test in detecting such subtle cognitive impairment. In addition to being strongly correlated with the overall MACFIMS score, its ability to classify patients as cognitively intact or not, with acceptable sensitivity (87%) and specificity (68%), justifies its clinical use. None of the scores obtained for the individual sub-sections reached such levels of sensitivity and specificity.
The MoCA test has a number of advantages: it is rapid, reliable and valid, and addresses many domains highly relevant to MS such as verbal memory and executive functions. These domains can be affected in some individuals who do not show impaired speed of information processing as assessed by the SDMT. Moreover, the MoCA test can easily be administered by most health professionals, is free of charge (although certification is now required, except for students, residents, fellows and neuropsychologists) and available in numerous languages directly through the Internet.
The multiple regression model demonstrates the importance of assessing executive functions, verbal fluency and verbal memory, as these were the functions that most effectively predicted the impairment revealed by the MACFIMS. These results are consistent with prior workReference Smith30 that showed that executive functions and verbal memory are the two aspects initially affected in MS and that they should be examined even in patients with a very low level of disability (EDSS ≤ 1.5).
These results also concur with those obtained by Vogel et al.,Reference Vogel, Banks, Cummings and Mille55 which showed that the visuospatial/executive, memory, attention and language domains of the MoCA test adequately reflected constructs similar to those measured by an exhaustive neuropsychological evaluation in a clinic specialised in neurodegenerative disease. The MoCA, which evaluates these functions, is, therefore, a more appropriate tool for screening in MS than other screening tests that do not evaluate these functions, such as the SDMT.
Our study has some limitations. It was conducted with rather young and educated patients and the range of MoCA test results observed was relatively limited (from 21 to 30). For improved generalisability, this study should be replicated among less educated and older patients, especially given the significant impact of these factors on cognition.Reference Sumowski, Wylie, Chiaravalloti and DeLuca56–Reference Scarmeas and Stern58
Using the MACFIMS as the gold standard, the MoCA test could accurately identify the presence of cognitive impairment in patients with MS without subjective cognitive complaints. This study suggests that the MoCA should be used more systematically in follow-up clinical appointments. Considering the impact of cognitive impairment on personal and professional life, this rapid screening test could be used to identify patients for whom a more detailed neuropsychological assessment would be recommended.
Acknowledgements
The authors would like to thank the patients who participated in this study. A special thanks to Hugues Leduc for his suggestion regarding the statistical analyses and to Karen Grislis for manuscript editing.
Disclosures
Dr Duquette has served on editorial boards, has been supported to attend meetings by EMDSerono, Biogen-Idec, Novartis, Genzyme, and TEVANeuroscience and received grants from CHIR and MS Society of Canada. The remaining authors have no conflicts of interest.
Statement of Authorship
KC: data analysis, manuscript writing and submission of the manuscript; AT: testing, statistical analyses and editing of the manuscript; RL: recruitment and testing. ER: study design and recruitment. PD: study design, recruitment and data analysis; IR: study design, recruitment and testing, data analysis, editing and submission of the manuscript.








