Rabies encephalomyelitis remains a difficult therapeutic challenge. To date, numerous therapies have been implemented with all failing to show therapeutic efficacy.Reference Fooks, Banyard, Horton, Johnson, McElhinney and Jackson 1 , Reference Jackson, Warrell and Rupprecht 2 Few reports of survivors exist in the literature, with the majority of survivors having received post-exposure prophylaxis with one or more doses of rabies vaccine.Reference Jackson 3 Documented survivors of rabies may, at least in part, represent advances in cardio-respiratory and other supports within modern critical care units and not be related to specific rabies directed therapies.
In 2004 a young patient from Wisconsin survived rabies and her therapy has been dubbed the Milwaukee protocol and relentlessly promoted.Reference Willoughby, Tieves and Hoffman 4 The Milwaukee protocol is a treatment regimen for rabies focused on therapeutic coma and the use of N-methyl D-aspartate (NMDA) receptor antagonist therapy. This protocol has received attention after recovery with mild neurological deficits of one patient who did not receive any rabies vaccine.Reference Willoughby, Tieves and Hoffman 4 , Reference Hu, Willoughby, Dhonau and Mack 5 Since the case report was published in 2005,Reference Willoughby, Tieves and Hoffman 4 many changes have been made in the protocol to arrive at its current rendition.Reference Willoughby 6
Critics of the Milwaukee protocol raise concerns of the regimen’s lack of efficacy in human rabies, with at least 31 documented failures reported in the literature to date (Table 1). There are claims that patients from ColombiaReference Caicedo, Paez and Kuzmin 19 , Reference Jackson and Garland 20 and PeruReference Aramburo, Willoughby and Bollen 12 who died are survivors because they survived the initial phase of acute illness. Another survivor probably did not have rabies,Reference Wiedeman, Plant and Glaser 39 whereas others received doses of rabies vaccine prior to the onset of their disease 40 similar to rabies survivors who did not receive the Milwaukee protocol. Given the concerns with the Milwaukee protocol, we elected to perform a critical appraisal of the suggested critical care directed therapies within the current protocol version, with a focus on NMDA receptor antagonism, therapeutic coma, and cerebral vasospasm.
Table 1 Cases of human rabies with treatment failures that used the main components of the Milwaukee protocol. (Updated with permission from Jackson AC: Therapy in human disease, in Rabies: scientific basis of the disease and its management, Third Edition, edited by AC Jackson, 2013, Elsevier Academic Press, Oxford, UK, pp 573-587; Copyright Elsevier.
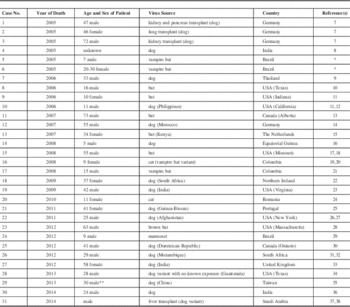
* Personal communication from Dr. Rita Medeiros, University of Para, Belem, Brazil.
** Patient was initially in a vegetative state but died within six months while in hospice care (Personal communication, Dr. Ya-Sung Yang, Tri-Service General Hospital, Taipei, Taiwan).
We reviewed the available literature regarding the treatment of human rabies encephalitis over the last decade and have summarized all known patients treated with the Milwaukee protocol to date. Furthermore, in order to outline any available evidence to support its use, we reviewed the literature surrounding the Milwaukee protocol for rabies encephalitis. Within the protocol we identified areas of concern for lack of scientific merit. These areas included therapeutic coma, NMDA receptor antagonism, and cerebral vasospasm prophylaxis/detection/treatment.
Method
Search strategy
We performed a review of the literature looking for therapy of all cases of human rabies published from 2005 (the inception of the protocol) to August 2015. MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and Cochrane Library from January 2005 to August 2015 were searched, using individualized search strategies for each database. Finally, reference lists of any review articles or cases on the management of human rabies were reviewed for relevant reports missed. Inclusion criteria were human subjects with rabies encephalitis, prospective or retrospective studies, any study size, and any language. Exclusion criteria were animal studies.
Study selection
A two-step review of all articles returned by our search strategies was performed. First, all titles and abstracts of the returned articles were screened to decide if they met the inclusion criteria. Second, the full text of the chosen articles was then assessed to confirm if they met the inclusion criteria.
Data collection
Data fields included patient demographics, region of origin, and outcome. We also documented the use of therapeutic coma, ketamine, amantadine, and ribavarin. Any cases describing the use of the main components of the Milwaukee protocol were added to the data from a previously published table from 2013.Reference Jackson 3 This was tabulated in Table 1. Of note, all 31 patients have died as a result of their illness due to rabies, including complications and related to neurological sequelae.
Results
The data from all patients from United States/ Canada/United Kingdom treated for rabies encephalitis from January 2005 to December 2014 are summarized in Table 2.
Table 2 Therapy in all 29 cases of human rabies occurring in the United States, Canada, and United Kingdom during the period 2005-2014. Twelve cases (41%) received major components of the Milwaukee protocol (therapeutic coma and ketamine). Therapy failed in all; the only survivor (Case No. 14) did not have neutralizing anti-rabies virus antibodies and likely did not have rabies,39 similar to Case No. 8, who did not receive any specific therapy and did not require critical care.41
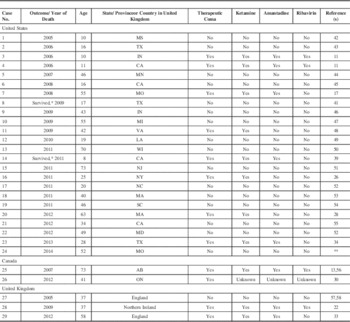
* Two patients recovered from an illness without the presence of neutralizing anti-rabies virus antibodies, raising very serious doubts about a diagnosis of rabies.
** Personal communication, Jesse Blanton, Center for Disease Control and Prevention, Atlanta, GA, USA.
A total of 29 patients with rabies encephalitis were treated during the described period. Only two patients treated during this period have been reported as survivors.Reference Wiedeman, Plant and Glaser 39 , Reference Holzmann-Pazgal, Wanger and Degaffe 41 However, in both of these cases the patients failed to develop neutralizing anti-rabies virus antibodies, raising serious doubts about whether these individuals actually had rabies encephalitis.
Appraisal of the Milwaukee Protocol
The current version of the Milwaukee protocol for the treatment of rabies viral encephalitis can be accessed online.Reference Willoughby 6 Table 3 summarizes the main components of the protocol.
Table 3 Summary of the Main Components of the Milwaukee Protocol, version 4.06

ICU=intensive care unit, DVT=deep vein thrombosis, NMDA=n-methyl d-aspartate, TCD=transcranial dopplers.
* Phenobarbital was used for maintain a burst-suppression pattern on the electroencephalogram for therapeutic coma for the index caseReference Willoughby, Tieves and Hoffman 4 .
NMDA Receptor Antagonism
Glutamate, the major stimulatory neurotransmitter in the central nervous system, mediates stimulation of NMDA receptors leading to neuronal calcium uptake, intracellular acidosis, nitric oxide synthase (NOS) mediated free radical formation, and mitochondrial dysfunction, which cause neuronal injury and death.Reference Lau and Tymianski 59 Concerns over glutamate mediated excitotoxicity in brain injury has led to the investigation of the neuroprotective effects of NMDA receptor antagonists.Reference Lau and Tymianski 59 - Reference Morris, Bullock, Marshall, Marmarou, Maas and Marshall 61 By antagonizing the NMDA receptor, the effects of the excitatory neurotransmitter glutamate can potentially be attenuated.
The use of NMDA mediated neuroprotection agents in traumatic brain injury (TBI)Reference Morris, Bullock, Marshall, Marmarou, Maas and Marshall 61 and strokeReference Di, Bullock and Watson 60 has led to mixed results to date. Both the TBI and stroke literature have displayed some benefit to NMDA receptor antagonism in animal models, but clinical trials have failed.Reference Morris, Bullock, Marshall, Marmarou, Maas and Marshall 61 , Reference Hoyte, Barber, Buchan and Hill 62 Amantadine, however, through a randomized trial in TBI has showed some benefit to functional outcome when administered within 4 to 16 weeks of injury.Reference Giacino, Whyte and Bagiella 63
In acute viral encephalitis, similar concerns over glutamate mediated excitotoxic neuronal damage have been raised.Reference Nargi-Aizenman, Havert, Zhang, Irani, Rothstein and Griffin 64 To no surprise NMDA receptor antagonists have been employed in both animal modelsReference Mori, Liu and Hossain 65 and human subjects.Reference Robertson, Clark and Markesbery 66 In Sindbis virus encephalomyelitisReference Nargi-Aizenman, Havert, Zhang, Irani, Rothstein and Griffin 64 , Reference Darman, Backovic and Dike 67 , Reference Nargi-Aizenman and Griffin 68 and human immunodeficiency virus infection,Reference Kaul and Lipton 69 there is established experimental evidence supporting excitotoxicity. However, this is lacking in rabies and in an animal model there is a lack of efficacy of ketamine therapy,Reference Weli, Scott, Ward and Jackson 70 which argues against this hypothesis. To date evidence for glutamate mediated excitotoxic neuronal damage in viral encephalitis is suggested in some animal models, and the implementation of NMDA receptor antagonists in human viral encephalitis is experimental. In corollary, literature is emerging suggesting that complete blockade of the NMDA receptor may have consequences on the normal inflammatory cascade and affect clearance mechanisms in bacterial infections, leading to impaired defense mechanisms.Reference Glezer, Zekki, Scavone and Rivest 71 This concern has yet to be confirmed in viral illnesses.
Milwaukee Protocol Recommendations
Within the current version of the Milwaukee protocol, reference to the use of NMDA receptor antagonists has been made due to speculation about a role of NMDA receptor mediated excitotoxicity and also their potential antiviral properties. Therapy with both ketamine and amantadine has been recommended.
Ketamine is a non-selective NMDA receptor antagonist that has been investigated for its antiviral properties in animal models of rabies.Reference Appolinario and Jackson 72 Both ketamine based inhibition of viral gene transcription and reduced tissue viral burden has been reported in a rat model,Reference Appolinario and Jackson 72 , Reference Lockhart, Tordo and Tsiang 73 but was not confirmed in more detailed and recent studies in a mouse model.Reference Luminos, Barboi and Draganescu 70 This antiviral effect of ketamine has not been demonstrated in any other model of acute viral encephalitis.
Ketamine, as a continuous infusion, has been recommended due to concerns over autonomic instability seen in human rabies.Reference Willoughby, Tieves and Hoffman 4 , Reference Willoughby 6 Ketamine has not been shown to be an effective neuroprotective agent in any neurological illness. Furthermore, outside of selected case reports, ketamine has not been studied as a therapy for autonomic instability.
Amantadine’s antiviral effects in rabies virus infection are based on limited in vitro studies and it failed to show efficacy in mice when inoculated into the site of intramuscular inoculation of wild-type rabies virus at daily intervals;Reference Appolinario and Jackson 72 it has not been evaluated in experimental rabies or in human rabies. Believed to inhibit rabies virus production, the current data suggest that amantadine likely does not impair viral attachment and penetration.Reference Appolinario and Jackson 72 Thus, the role for amantadine as an antiviral agent in rabies is unclear and it cannot be recommended at this time. Similarly, the neuroprotective role of amantadine to date has been only demonstrated in the TBI literature.Reference Giacino, Whyte and Bagiella 63 No data exists to suggest its efficacy in human viral encephalitis.
Given the failure of NMDA receptor antagonist therapy for neuroprotection in a variety of neurological disorders, the lack of evidence supporting its antiviral and neuroprotective properties in rabies and other forms of viral encephalitis, rabies models questioning a pathogenetic role of excitotoxicity,Reference Weli, Scott, Ward and Jackson 70 and a lack of evidence supporting the use of NMDA receptor antagonist therapy for autonomic instability, the current recommendations for ketamine or amantadine therapy within the Milwaukee protocol cannot be supported.
Therapeutic Coma
Therapeutic coma involves the administration of high dose anesthetic agents in order to achieve maximal metabolic suppression and neuronal preservation. This is typically achieved using high dose intravenous sedative agents, however, the indications for such therapy are unclear. This therapy has been described in TBI, status epilepticus, stroke, and cardiopulmonary bypass animal models displaying neuronal preservation.Reference Schifilliti, Grasso, Conti and Fodale 74
The use of therapeutic coma in neurocritical care is controversial. Concerns with implementing aggressive sedation focus around monitoring the depth of anesthesia, goals of anesthesia, type of anesthestic agent to use, duration of therapy, and the complications associated with such therapy. Furthermore, aggressive sedative regimens, including targeting burst suppression, have failed to display an impact on patient outcome in TBIReference Roberts and Sydenham 75 and are not generally used for other acute neurological diseases. Therapeutic coma in the setting of viral encephalitis has no support within the literature and has only been recommended in a small number of cases with refractory intracranial hypertension or status epilepticus.Reference Kramer 76
Within the critical care literature in general, excessive sedation is also a hot topic. Recent literature has documented the association between the amount of sedation and mortality.Reference Shehabi, Chan and Kadiman 77 Unfortunately, the majority of these studies excluded patients with neurological illness. Regardless, unnecessary sedative use should always be avoided in all critically ill patients.
The use of therapeutic coma in rabies currently is not based on scientific evidence and there have been many documented failures. The Milwaukee protocol also justifies sedation therapy in order to prevent the fatal consequences related to autonomic instability in rabies. The incidence of autonomic instability in rabies encephalitis is unknown, although it has been frequently reported. Many accounts are not detailed enough to make this determination on a case by case basis. Autonomic instability may be secondary to severe critical illness and multi-organ dysfunction (MOD),Reference Wieske, Chan Pin Yin, Verhamme, Schultz, van Schaik and Horn 78 , Reference Schmidt, Hoyer and Wilhelm 79 or to rabies virus infection involving the autonomic nervous system. Therapeutic coma is currently not a well described nor generally accepted therapy for autonomic dysfunction. The origins of the recommendation surrounding therapeutic coma in the Milwaukee protocol are unclear and may in fact stem from early reports of improved survival of patients with encephalitis treated with sedation. The index case had neutralizing anti-rabies virus antibodies at the time of presentation,Reference Willoughby, Tieves and Hoffman 4 which is thought to be important for subsequent viral clearance and survival. Her survival was likely related to her individual inherent resistance to the viral infection and modern supportive critical care but not actually the therapeutic coma itself.
Inducing coma via intravenous anesthetic agents impairs the neurological examination, leads to vasopressor dependency, increases the risk of infections, and is associated with intensive care unit mortality and thus should be avoided.Reference Shehabi, Chan and Kadiman 77 The impact of aggressive sedative regimens on the disease course in rabies and patient outcome are unknown. However, given the breadth of data available from the general critical care literature, caution should be taken with over sedation in patients with rabies.
Cerebral Vasospasm
Pathophysiology
Cerebral vasospasm leading to delayed cerebral ischemia (DCI) is commonly described in the literature following aneurysmal subarachnoid hemorrhage (SAH) and in TBI. Thus, the majority of literature on monitoring and therapies for DCI secondary to cerebral vasospasm is based on data from these populations. Cerebral vasospasm has not been generally recognized in viral encephalitis and there is no clinical or pathological evidence supporting its occurrence in this setting, including in rabies.
Currently, few cases of imaging/radiographic cerebral vasospasm in rabies have been described.Reference Willoughby, Roy-Burman and Martin 80 , Reference Sing and Soo 81 There have not been any cases of symptomatic DCI secondary to cerebral vasospasm reported in human rabies. Mechanistically, cerebral vasospasm in the setting of SAH and TBI is thought to be related to an inflammatory response secondary to blood breakdown products in contact with the vessel adventitia. In addition, the role of microthrombosis, microvascular spasm, and failure of autoregulation in cerebral vasospasm have been questioned.Reference Budohoski, Guilfoyle and Helmy 82
In rabies this theory of vasospasm does not apply. It is hypothesized that, in rabies, there is a loss of nitric oxide synthase (NOS) function secondary to an induced tetrahydrobiopterin deficiency and this NOS dysfunction, in concert with inflammation, can lead to cerebral vasospasm.Reference Willoughby, Opladen and Maier 83 This theory has yet to be substantiated. Furthermore, with the lack of human clinical descriptions and neuropathological studies in rabies supporting the presence of cerebral vasospasm, it is unlikely that it plays any important role in rabies encephalitis.
Monitoring
Monitoring of cerebral vasospasm is a contentious topic.Reference Marshall, Nyquist and Ziai 84 Recommendations for regular monitoring via transcranial Doppler (TCD), computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) have been made in the literature.Reference Marshall, Nyquist and Ziai 84 Given the speed and ability to perform at the bedside, TCD monitoring has been recommended as a screening tool for vasospasm in both SAH and TBI.Reference Marshall, Nyquist and Ziai 84 However, there are concerns with inter-/intra-operator variability of the results, limitations in technique due to patient specific cranial windows, and concerns on reliability of readings for non-middle cerebral artery vessels.
The Milwaukee protocol recommends regular TCD monitoring for vasospasm in rabies. Given the current controversy, this strong recommendation cannot be supported.Reference Willoughby 6 In addition, given the recommendation of an aggressive sedative regimen for rabies patients within the protocol, the utility of screening patients with TCD for vasospasm in this context is questionable because the indication for treatment is based on the presence of clinical examination changes, which may, or may not, represent the features of DCI. In the setting of therapeutic coma, as recommended by the Milwaukee protocol, the treating intensivist’s ability to determine if TCD defined vasospasm is clinically relevant is dramatically impaired.
The Milwaukee protocol defines treatable vasospasm as those patients with either a bilateral increase in TCD based middle cerebral artery (MCA) velocities with normal resistance or a bilateral decrease in MCA velocities with increased resistance.Reference Willoughby 6 The exact cutoff for “increased” or “decreased” MCA velocities is not described and no reference to standard TCD definitionsReference Rigamonti, Ackery and Baker 85 of cerebral vasospasm in aneurysmal SAH are made. The potential adverse consequences of implementing hypertensive therapy or vasodilator therapy for TCD defined vasospasm in rabies, without recognized clinical consequences, should not be ignored.
In addition to TCD monitoring, the Milwaukee protocol recommends continuous electroencephalogram (cEEG) monitoring for DCI secondary to cerebral vasospasm.Reference Willoughby 6 It has been suggested that the presence of spreading depolarizations may be an indication of underlying DCI.Reference Hanggi 86 However, recent literature indicates a lack of large prospective studies utilizing cEEG monitoring in aneurysmal SAH patients, and does not support widespread use of this modality as a monitor for DCI secondary to cerebral vasospasm.Reference Hanggi 86 Thus, the recommendation of its use in rabies encephalitis as a monitor for DCI is clearly not warranted.
Prophylaxis for Vasospasm
Prophylaxis of vasospasm has also been recommended within the Milwaukee protocol. The use of nimodipine, vitamin C, saproterin and L-arginine have been recommended within the most recent version of the protocol.Reference Willoughby 6
Nimodipine, a calcium channel antagonist, has been widely used in SAH patients as a means of prophylaxis against cerebral vasospasm and is currently considered standard care in this patient population.Reference Dorhout Mees, Rinkel and Feigin 87 In the TBI population, where cerebral vasospasm is also prevalent, nimodipine has failed in large trials to have an impact.Reference Langham, Goldfrad, Teasdale, Shaw and Rowan 88 Hence, the recommendation of nimodipine to prevent rabies associated vasospasm is unsubstantiated.
Vitamin C therapy has been investigated as a potential means to prevent cerebral vasospasm in aneurysmal SAH by altering the activity of oxyhemoglobin.Reference Kawakami, Kodama and Toda 89 This benefit has not been demonstrated in human subjects. Regardless, vitamin C therapy has been included in the protocol. Furthermore, a small reports have eluded to an “antiviral” effect of vitamin C in rabies models.Reference Banic 90 This effect, again, has not been supported in human reports.
Sapropterin supplementation has been suggested, given the concerns of tetrahydrobiopterin deficiency leading to NOS dysfunction and potentiation of cerebral vasospasm.Reference Willoughby, Opladen and Maier 83 No data exist to suggest that supplementation leads to a decrease in cerebral vasospasm or improvement in outcome.
Finally L-arginine has also been recommended. L-arginine is postulated to induce NOS function and promote vasodilation.Reference Perko, Pretnar-Oblak, Sabovic, Zvan and Zaletel 91 The use of L-arginine to prevent or treat cerebral vasospasm has not been supported by the literature, thus its recommended use in the treatment of rabies should be cautioned.
Treatment
Currently, the management of cerebral vasospasm, regardless of the underlying neurologic cause, is based on literature from aneurysmal SAH patients. The current “gold standard” therapy for symptomatic DCI post aneurysmal SAH is hypertension therapy, with the goal of raising mean arterial pressures (MAP) in order to preserve cerebral blood flow (CBF) in at risk territories,Reference Diringer, Bleck and Claude 92 Previous therapies consisted of hypertension, hypervolemia and hemodilution, termed “Triple-H” therapy.
Treatment of TCD or CTA defined cerebral vasospasm, without a clinical correlate of symptomatic DCI has also been recommended by the Milwaukee protocol.Reference Willoughby 6 The Milwaukee protocol references “Triple-H” therapy as the treatment for TCD defined vasospasm in rabies encephalitis. This is concerning considering the evidence in the SAH literature warning against hypervolemia and hemodilution therapy.Reference Diringer, Bleck and Claude 92
In addition to triple-H therapy, the Milwaukee protocol recommends continuous intravenous infusions of nicardipine for TCD defined cerebral vasospasm. This is currently not recommended as standard care in any guidelines for the management of cerebral vasospasm.Reference Diringer, Bleck and Claude 92 To date, only a small number of studies in aneurysmal SAH have studied nicardipine, displaying an improvement in radiographic vasospasm,Reference Velat, Kimball, Mocco and Hoh 93 without a robust impact on outcome. Its recommendation in rabies is unwarranted.
Overall, the treatment recommendations made by the Milwaukee protocol for cerebral vasospasm in rabies encephalitis are not the current standard care for vasospasm in other neurological disorders and are unwarranted.
Conclusions
Despite initial hope and enthusiasm for the Milwaukee protocol in the treatment of rabies, subsequent trials of this regimen have failed. Serious concerns over the current protocol recommendations are warranted in light of a weak scientific rationale. The recommendations for therapeutic coma, NMDA receptor antagonists, and the screening/prophylaxis/treatment of cerebral vasospasm are supported by little to no scientific evidence in the literature. The recommendations made by the protocol warrant serious reconsideration before any future use of this failed protocol. Unfortunately, we do not have an alternative protocol to put forward for therapy of patients with rabies. We hope that novel therapies will be developed after we have an improved understanding of rabies pathogenesis and further research in good animal models is very important. We have recently published a detailed review of antiviral therapy in rabiesReference Appolinario and Jackson 72 and potential approaches to management,Reference Jackson 94 including the possibility of regional (head and neck) cooling (hypothermia).
DISCLOSURES
Frederick Zeiler and Alan Jackson do not have anything to disclose.





