In patients with acute ischemic stroke (AIS), collaterals provide blood flow to tissue at risk via arterial backfilling. Good collateral status measured on computed tomography angiogram (CTA) is associated with improved imaging and clinical outcome.Reference Christoforidis, Karakasis, Mohammad, Caragine, Yang and Slivka 1 - Reference Souza, Yoo and Chaudhry 4 Various imaging modalities are used to measure collateral status in patients with acute ischemic stroke. Magnetic resonance angiography and transcranial Doppler are noninvasive techniques; however, they are logistically challenging and may not be available at all centres. Alternatively, the reference standard, the four-vessel digital subtraction angiogram (DSA), is invasive. Finally, CTA is readily available at many centres, and only adds minutes to the noncontrast CT acquisition.
For an imaging modality to be considered as a reasonable standard for measuring collateral status, it must have biological validity. In a previous study, Menon et alReference Menon, O’Brien and Bivard 5 found significant variability in collateral status within the middle cerebral artery (MCA) ischemic region. The anterior cerebral artery (ACA)-MCA collaterals behaved differently to the posterior cerebral artery (PCA)-MCA collaterals. This regional variance in collateral status correlates well with brain tissue viability and therefore clinical outcomes. The current reference standard for collateral assessment (i.e. American Society of Interventional and Therapeutic Neuroradiology [ASITN] score on conventional angiogram) or other methods of assessment of collaterals on CTA do not account for this regional variation in collateral status. Of note, collateral assessment on conventional angiograms is almost always restricted to scoring collaterals on images obtained from single arterial injections into the ipsilesional common carotid or internal carotid artery, thus not measuring PCA-MCA collaterals.
In this study, we measure ACA-MCA and PCA-MCA pial filling independently on single-phase CTA (sCTA)Reference Garcia-Tornel, Carvalho and Boned 6 and correlate it with the CTA-based Massachusetts General Hospital (MGH)Reference Souza, Yoo and Chaudhry 4 and digital subtraction angiography (DSA)-based ASITN collateral score.Reference McVerry, Liebeskind and Muir 7 We hypothesize that ACA-MCA collaterals (as is done by the ASITN score on conventional angiogram and to a lesser extent by the MGH score on CTA) only capture a small proportion of the variance in PCA-MCA collateral extent.
Methods
Patient imaging data were collected from the Calgary Identifying Novel Approaches to Optimize Arterial Imaging Interpretation for Predicting and Measuring Recanalization Whatever the Treatment and to Optimize Parenchymal Imaging Interpretation for Prediction of Early Neurological Recovery After Recanalization Using Serial CT Angiography prospective imaging registry. Each patient underwent a baseline noncontrast CT and CTA . Patients selected to undergo endovascular therapy had DSA. Noncontrast CT was obtained with a 5-mm slice thickness and 140 kVP. Next, CTA imaging was acquired from aortic arch to vertex (1-mm slice thickness, 120 kVP) using a 100-ml bolus of Optiray 320 contrast.
A total of 106 patients with AIS and a proximal occlusion were included in our study. Each of these patients presented with an M1 MCA±intracranial ICA occlusion on baseline CTA. Phase of image acquisition on baseline CTA was assessed in accordance with the previously published methodology by Rodriquez-Luna et alReference Rodriguez-Luna, Dowlatshahi and Aviv 8 (Table 1). The phase (early, peak arterial, equilibrium, early, late venous) was determined by evaluating Hounsfield units (HU) on the contralateral (unaffected) vasculature at the ICA, M1 MCA, superior sagittal sinus, torcula, and transverse/sigmoid sinus. The contralateral vasculature was chosen to measure the HU to minimize the potential impact from the proximal occlusion on the ipsilateral side.
Table 1 HU thresholds defining phase of image acquisitionReference Riva, Pappada and Papadakis 17
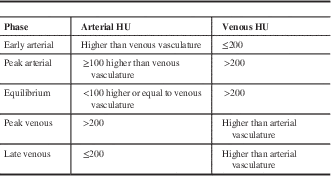
HU=Hounsfield unit.
Collaterals beyond the M1 MCA occlusion were assessed using the Calgary Collateral (CC) Score (Table 2). This score measures pial arterial filling in ACA-MCA and PCA-MCA regions separately on a 5-point ordinal scale (Figure 1). These scores are then added to obtain a 10-point score. Additionally, the evaluator assessed collateral status on CTA and DSA using the MGH score and ASITN score. These scores measure collateral status in the entire ischemic region. These scores are also described in Table 2. Quantomo (Cybertrial Inc., Calgary), a validated software tool, was used to measure infarct volume in millilitres on 24- to 48-hour follow-up MRI (or CT when MRI was not available) while being blinded to all other clinical and imaging information. Manual adjustments to delineate infarct boundaries were performed where necessary. If the infarct showed haemorrhagic conversion, the hemorrhage regions were incorporated within the boundaries of infarct.
Table 2 The Calgary Collateral Score, the ASITN score on DSA and the MGH score
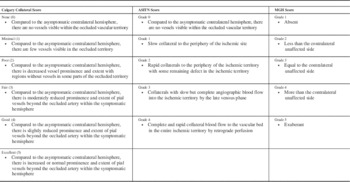
Note the Calgary Collateral score is scored separately in the ACA-MCA and PCA-MCA regions for a total score of 10.Reference Souza, Yoo and Chaudhry 4 , Reference Menon, D’Esterre and Qazi 13
The three scores were compared with each other using nonparametric statistics (Spearman’s correlation coefficient rho [rho]). Linear regression was used to assess the relationship between collateral status and infarct volume on follow-up after verifying if relevant statistical assumptions were met. Information theory approaches (Akaike [AIC] and Bayesian Information Criterion [BIC]) were used to determine which CTA model best predicted follow-up infarct volumes.
Results
A total of 106 patients were included in the analysis. Baseline sCTA was acquired in early arterial phase in 9.9%, peak arterial in 50.7%, equilibrium in 32.4%, early venous in 5.6%, and late venous in 1.4% (Figure 2). Scans acquired in the very early arterial phase or late venous phase were excluded from further analysis.
A modest correlation was observed between ACA-MCA and PCA-MCA collaterals using the CC Score. Only 32% of the variance in PCA-MCA collaterals was explained by ACA-MCA collaterals (rho=0.56, p<0.0001).
Correlation between ACA-MCA collaterals and the CTA-based MGH score was strong (rho=0.8, p<0.0001); correlation between PCA-MCA collaterals and this score, however, was modest (rho=0.54, p<0.0001). Correlation between ACA-MCA collaterals and the DSA based ASITN score was modest (n=53, rho=0.43, p=0.16) but correlation among PCA-MCA collaterals and ASITN score was poor (rho=0.33, p=0.4).
Infarct volume on follow-up scans was measured in 90/106 patients. Mean infarct volume was 51.8 ml (standard deviation, 82.3 ml). On linear regression, a statistically significant relationship was noted between the CC Score and follow-up infarct volume (p<0.001) and between the MGH score and follow-up infarct volume (p<0.001). The statistical model that used the CC Score (AIC, 1022; BIC, 1027) was a better fit at predicting follow-up infarct volumes than the MGH score (AIC, 1029; BIC, 1034). Relationship between ACA-MCA and PCA-MCA collateral scores and model derived “predicted follow-up infarction volume” is shown in Figure 3.
Discussion
Leptomeningeal collaterals act as a system to unify the major cerebral arterial systems and provide a mechanism for retrograde blood flow during AIS. Our results substantiate previous findings showing significant variability in collateral status between ACA-MCA and PCA-MCA collaterals in patients with acute anterior circulation ischemic strokes with M1 MCA occlusions. We also show scoring collaterals on conventional angiograms (using the ASITN score) or on CTA using scores that look at the MCA region as a whole do not capture this regional variability in collateral status. Our results thus support the importance of assessing regional variability in collateral status while scoring them using CTA. Finally, we show that CTA-based collateral scores that take into account regional variability in pial arterial filling are better in predicting follow-up infarct volumes than scores that do not.
Assessing regional variability in collateral status is clinically relevant because it likely helps clinicians predict posttreatment regional tissue fate in a better manner. Moreover, improving methods of assessing collaterals on CTA is important because collaterals affect clinical decision-making in acute ischemic stroke by predicting tissue fate and clinical outcomes.Reference Goyal, Demchuk and Menon 9 , Reference Leng, Lan, Liu, Leung and Wong 10 Good collateral scoring appears to be associated with better clinical prognosis in patients with AIS.Reference Tan, Wan-Yee and Paliwal 11 Alternatively, poor collaterals are associated with poorer outcomes and even hemorrhagic transformation.Reference Brunner, Tomandl, Hanken, Hildebrandt and Kastrup 12
Our results show that approximately 11.5% of sCTA scans are acquired in the very early arterial phase or the late venous phase. As noted in previous studies, mistiming of CTA acquisition, especially with sCTAs, can result in mislabelling of collateral status.Reference Menon, D’Esterre and Qazi 13 , Reference Menon, Smith and Modi 14 Rate of contrast injection, time between contrast injection and image acquisition, and patient factors, including low cardiac ejection fraction, may introduce variability in assessment of collateral assessment with sCTA.Reference Brunner, Tomandl, Hanken, Hildebrandt and Kastrup 12 , Reference Soares, Tong and Hom 15 sCTA may capture anterior dominant patterns before the flow of contrast is sufficient in the posterior collateral circulation for collateral assessment; PCA-MCA filling may be slower than the ACA-MCA territory.Reference Arsava, Vural and Akpinar 16 These deficiencies with sCTA can be addressed to a significant extent using multiphase CTA.Reference Menon, D’Esterre and Qazi 13
Limitations of our study include relatively small sample size and the use of a single evaluator trained in the use of MGH, ASITN, and CC Scores.
Conclusion
Collateral assessments in patients with acute ischemic stroke are best done using CTA, preferably with adequate temporal resolution and by assessing regional variability. Moreover, ACA-MCA and MCA-PCA collaterals should be evaluated separately because the ASITN score and MGH score may not account for collateral regional variability.
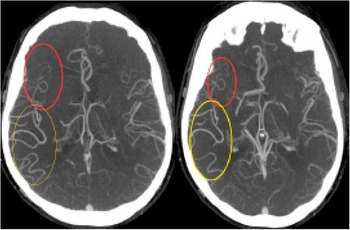
Figure 1 Single phase computed tomography angiography demonstrating posterior cerebral artery (PCA)-middle cerebral artery (MCA) dominant collateral pattern in a patient with M1 MCA occlusion. In addition to exuberant MCA-PCA filling (yellow circle), there is poor anterior cerebral artery-MCA filling seen (red circle).
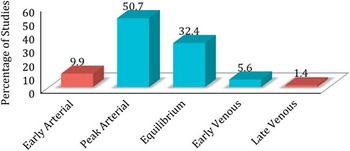
Figure 2 The proportion of patients separated by phase of single phase computed tomography angiography acquisition (early arterial, peak arterial, equilibrium, early venous, late venous) in our study.
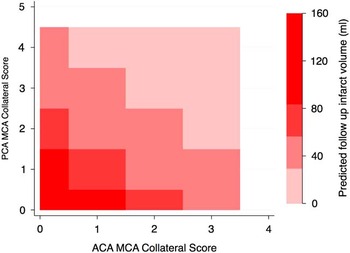
Figure 3 Contour map showing predicted infarct volume on follow-up imaging estimated using anterior cerebral artery (ACA)-middle cerebral artery (MCA) collateral score (x axis) and posterior cerebral artery (PCA)-MCA collateral score (y axis).
Acknowledgements and Funding
The authors thank the University of Calgary Department of Clinical Neurosciences, the Hotchkiss Brain Institute, and the Calgary Stroke Program for their ongoing support, and the reviewers at Canadian Journal of Neurological Sciences for their hard work and consideration.
The Identifying Novel Approaches to Optimize Arterial Imaging Interpretation for Predicting and Measuring Recanalization Whatever the Treatment and to Optimize Parenchymal Imaging Interpretation for Prediction of Early Neurological Recovery After Recanalization Using Serial CT Angiography (INTERRSeCT) study was funded through a grant from the Canadian Institute of Health Research (CIHR). BKM holds the current Heart and Stroke Foundation/University of Calgary Professorship in Stroke Imaging and a CIHR New Investigator Award.
Disclosures
BKM reports the following disclosures: Canadian Institute of Health Research (CIHR): grant recipient, CIHR grant, New Investigator Award recipient, CIHR award. Heart and Stroke Foundation: professorship, and award. CC, AT, SIS, EQ, ASAL, MB, CD, MA, AMD, and MG have nothing to disclose.







