Acute invasive fungal rhinosinusitis (AIFR) is a life-threatening condition that predominantly affects immunocompromised patients. Early symptoms of the disease include fever, nasal obstruction, and rhinorrhea.Reference Payne, Mitzner, Kunchala, Roland and McGinn 1 Head imaging shows asymmetric mucosal paranasal sinus thickening, sinus opacification, and periantral fat obliteration;Reference Aribandi, McCoy and Bazan 2 , Reference Gillespie, Huchton and O’Malley 3 intracranial and intra-orbital extension of infection are common, and contrast enhancement may be patchy, with focally non-enhancing areas indicating necrosis.Reference Aribandi, McCoy and Bazan 2 Endoscopic biopsy of sinus tissue is important and may be diagnostic of AIFR; however, the presence of fungal elements on middle turbinate biopsy—although 100% specific—is only 75% sensitive for AIFR.Reference Gillespie, Huchton and O’Malley 3 Because several other conditions can mimic AIFR, the interpretation of a negative middle turbinate biopsy presents a diagnostic challenge.
A 72-year-old woman with longstanding diabetes mellitus also had a 10-year history of splenomegaly with occasional peripheral blast cells; prior bone marrow examination was non-diagnostic. One month before presentation at our hospital, she was admitted to an outside hospital with headache, vomiting, and a swollen, red right eye. At that time her pupils and ocular movements were normal. One week later her left eye became painful and swollen. Methylprednisolone was started to try to reduce her orbital swelling, treating a presumed allergic reaction. Five days before coming to our hospital, her ocular symptoms worsened. She had no fever and no diplopia.
She was transferred to our hospital, at which time she had complete right-sided ptosis and near-complete left-sided ptosis. Her visual acuity was hand-motion-only in the right eye and with the left eye she did not have light perception. She had complete right-sided ophthalmoplegia while her left eye retained a limited ability to abduct. She had bilateral proptosis and conjunctival edema. She had diminished bilateral facial pinprick sensation and a slight right-sided facial droop. Head MRI revealed marked, symmetric paranasal sinus enhancement with periorbital swelling (Figure 1). There were no necrotic areas seen. Her leukocyte count was 42×109/L, hemoglobin was 98 g/L, platelets were 53×109/L, neutrophils were 32×109/L, and monocytes were 8.4×109/L (20% of total leukocytes), with no blasts identified. Her platelet count dropped further, despite a platelet transfusion, so lumbar puncture was not attempted. Given her history of diabetes, steroid use, rapidly progressive cranial neuropathies (II through VII), and proptosis, there was clinical concern for AIFR. She was started on empiric meropenem, vancomycin, and amphotericin B. She underwent nasopharyngoscopic examination, which was unremarkable: her nasal mucosa appeared normal, bled appropriately, and had normal sensation. A middle turbinate biopsy was performed, and histopathological examination of the specimen showed no fungal elements. Despite broad-spectrum antimicrobial therapy, she rapidly developed renal, respiratory, and bone marrow failure and died on her 4th day after admission.
An autopsy was performed. Her bone marrow was hypercellular with myeloid hyperplasia and increased monocytes consistent with chronic myelomonocytic leukemia (CMML). Her World Health Organization subtype of CMML was CMML-0.Reference Arber, Orazi and Hasserjian 4 Her International Prognostic Staging System CMML score was 1.0, Intermediate-1 category. Her myelofibrosis (MF) grading was MF2 (out of 3). Monocytic cells, neutrophils, and early myeloid cells had also infiltrated her periorbital soft tissue, retinas, sclerae, and leptomeninges (Figure 2). All cultures and special stains of sampled tissue were negative for fungal elements.
This woman had clinical features that put her at risk for AIFR, but endoscopic biopsy of sinonasal tissue was negative. A false negative biopsy was possible, as sensitivity is only 75%; however, clinical and laboratory features increased the probability that the negative biopsy result was a true negative.Reference Gillespie, Huchton and O’Malley 3 Specifically, she had no history of diabetic ketoacidosis; she had no fever; she had an elevated—not reduced—neutrophil count; and her imaging revealed symmetric pansinus enhancement.
We strongly suspect that her renal, respiratory, and bone marrow failure was because of her CMML or complications directly related to CMML. The amphotericin that she received may have contributed to her renal failure. Some histopathological changes could have been confounded by the steroids that she received near the end of her life.
Mimics of AIFR can be divided into vasculitic, infectious, neoplastic, and other causes. Vasculitic etiologies include granulomatosis with polyangiitis (GPA) and eosinophilic GPA.Reference Montone 5 , Reference Aoyama, Ishikura and Tsumura 6 Infectious mimics of AIFR include bacterial and parasitic disease.Reference Montone 5 , Reference Aoyama, Ishikura and Tsumura 6 Neoplastic mimics can be divided into primary cancers, such as sinonasal carcinoma; metastatic cancer, such as breast cancer; and hematologic cancers, such as lymphoma and leukemia.Reference Montone 5 - Reference Malecha and Holland 8 There have been at least four confirmed reports of central nervous system spread of CMML.Reference Aoyama, Ishikura and Tsumura 6 - Reference Malecha and Holland 8 Finally, the “other” mimics of AIFR include sarcoidosis and cocaine-induced midline destructive disease.Reference Montone 5
It is imperative that any immunocompromised patient who presents with rapidly progressing multiple cranial neuropathies be worked up for AIFR. The high mortality of this entity mandates a high index of suspicion for fungal disease, even when the middle turbinate biopsy is negative. A history of peripheral leukocytosis, symmetric pansinus enhancement, and bone marrow infiltrate are all features that point toward hematologic malignancy as the cause of biopsy-negative acute ophthalmoplegia. The progressive ophthalmoplegia and multiple cranial neuropathies that occurred in our patient with CMML are an illustration for other neurologists of just how closely CMML can mimic AIFR.
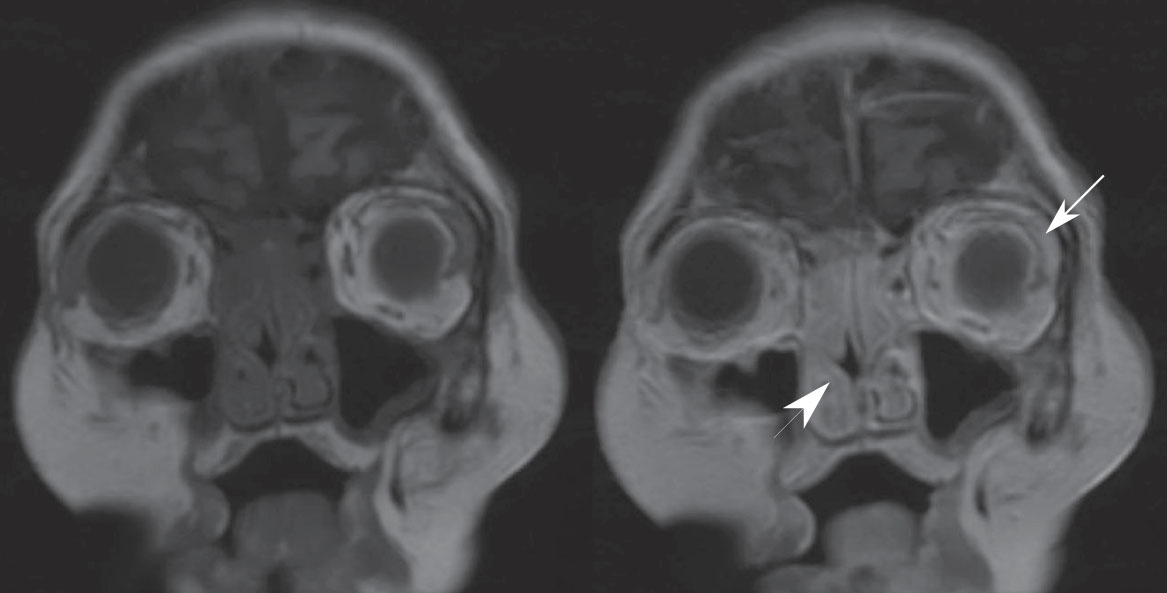
Figure 1 Coronal T1-weighted MRI without (left) and with (right) gadolinium of a 72-year-old diabetic woman with rapidly progressive multiple cranial neuropathies and proptosis. There is symmetric pansinus enhancement (arrowhead) with periorbital tissue swelling and enhancement (arrow). The extraocular muscles appear normal. The cavernous sinuses and pachymeninges enhance symmetrically, although there is no leptomeningeal enhancement (not pictured). No areas of necrosis are seen.
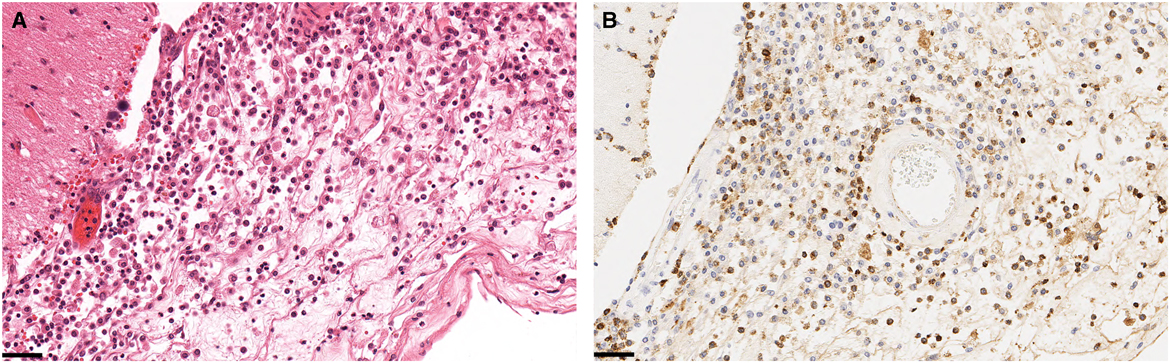
Figure 2 Histologic specimens taken at autopsy of the periorbital soft tissues and leptomeninges. Hematoxylin and eosin staining (A) shows monocytic cells, histiocytes, and neutrophils. Adjacent tissues with myeloperoxidase stain (B) reveal myeloid cells, consistent with infiltration by chronic myelomonocytic leukemia. The black scale bars are 50 μm. No fungal elements are seen with Grocott’s methenamine silver stain or periodic acid–Schiff–diastase special stains (not shown).
Acknowledgments
The authors thank the patient and her family.
Conflicts of Interest
None.
Disclosure
SC, MC, CH, LB, MS, KR, and JAF have nothing to disclose.
Financial Support
None.
Statement of Authorship
SC was involved in manuscript concept and case review. MC, CH, LB, MS, KR, and JAF contributed to critical revision of the manuscript for intellectual content. All authors approved the final version of the manuscript to be published.




