Atrial fibrillation (AF), an independent predictor for ischemic stroke, increases the risk by three to five fold. Reference Kannel, Wolf, Benjamin and Levy1 Oral anticoagulation (OAC) is an effective treatment to prevent ischemic stroke when compared to placebo or antiplatelet therapy. Reference Ruff, Giugliano and Braunwald2,Reference Hart, Benavente, McBride and Pearce3 Nonetheless, a residual stroke risk despite OAC remains, estimated at 1.4% per year with direct oral anticoagulants (DOAC) use and 1.7% per year with vitamin-K antagonists (VKA). Reference Ruff, Giugliano and Braunwald2 A recent pooled analysis showed that patients with ischemic stroke despite OAC are at higher risk of recurrent events, thereby highlighting the need to optimize stroke prevention strategies in this patient population. Reference Seiffge, De Marchis and Koga4 Mechanisms underlying ischemic stroke despite therapeutic OAC remain however largely elusive.
Modifiable causes of ischemic stroke despite OAC include pharmacological inefficacy due to either patient noncompliance, inappropriate dosing, drug or food interactions or periprocedural interruption. Reference Andrade, Aguilar and Atzema5 Furthermore, the presence of other stroke mechanisms (such large or small-vessel disease) may contribute to ischemic stroke risk. Reference Purrucker, Hölscher, Kollmer and Ringleb6,Reference Freedman, Martinez, Katholing and Rietbrock7 Beyond easily-identifiable stroke mechanisms, however, a significant proportion of ischemic strokes despite OAC remain unexplained and portend worse clinical outcomes. Reference Seiffge, De Marchis and Koga4 Due to a lack of available evidence to guide management, specific recommendations can not be made regarding optimal management in these patients. Reference Andrade, Aguilar and Atzema5,Reference Gladstone, Lindsay and Douketis8 The aim of this study was to describe the characteristics of patients presenting with an ischemic stroke despite OAC use in a single high-volume Canadian comprehensive stroke center (CSC).
A retrospective observational study was performed of consecutive patients evaluated for acute stroke at the Centre Hospitalier de l’Université de Montreal, with clinical data prospectively collected in the Montreal Neurovascular and STrokE Repository. A large proportion of suspected acute stroke patients arrive at our center after redirection from primary stroke centers (PSC) by paramedics in the case of severe suspected stroke. As such, redirected patients are repatriated to PSC the next day to continue medical management, including stroke workup. All consecutive adults diagnosed with acute ischemic stroke and preexisting use of OAC for known AF between 12/01/2017 and 03/31/2021 were included in the study. Data were analyzed separately as: 1) the whole cohort (all stroke patients evaluated at the CSC) and 2) local cohort (patients subsequently hospitalized at our institution), on account of missing data, and in particular in-hospital stroke workup, in the subgroup of patients subsequently repatriated to PSC. Data collection and analyses was approved by the local institutional review boards with waiver of patient consent given the retrospective nature of the study (local REB project number: 2021-9429, 20.337). Statistical analyses included chi-square test of independence or Fisher’s exact tests for categorical variables and the Mann–Whitney U-test for continuous variables to test differences between groups. Outcomes were dichotomized into favorable (mRS ≤ 2) and poor outcome (mRS 3–6). Data were analyzed using SPSS software (IBM SPSS Statistics Version 26.0.0.1). Statistical level of significance was set at p < 0.05.
During the study period, 2,700 patients were evaluated for a suspected acute stroke, of which 173 (0.64%) were diagnosed with an ischemic stroke despite OAC and were included in the study (whole cohort). Among these, 65 patients were subsequently hospitalized at the CSC (local cohort). Baseline characteristics including prior antithrombotic use for the whole cohort are shown in Table 1. Regarding OAC at the time of stroke, 27 (15.6%) patients were on VKA, while 146 (84.4%) were prescribed DOACs. Suboptimal OAC treatment was found in 52 (30%) patients, due to inappropriate dosing (17%), and patient non-adherence (13%). A concomitant stroke mechanism was found in 19 (11.0%) patients, and stroke etiology was classified as undetermined other than AF in 77 (44.5%) patients. An interruption of the OAC treatment was present in 25 (14.5%) patients, on account of bleeding complications in 3, recent stroke in 2, and invasive procedures in 20 patients including gastrointestinal investigations, dental procedures, cardiac pacemaker change, skin biopsy, and ENT surgery). Data regarding timing and duration of periprocedural OAC interruption were unavailable.
Table 1: Baseline characteristics of the whole study cohort (n = 173) and the hospitalized (local) subgroup (n = 65) with acute ischemic stroke despite therapeutic anticoagulation. Values are presented as n (%), mean ± SD or median [IQR]
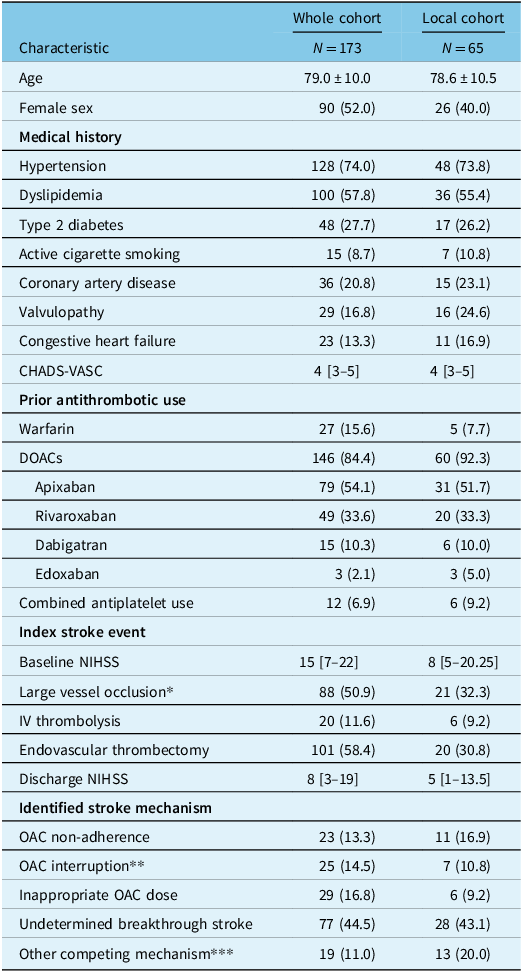
* CTA was not performed at presentation in 26 patients (whole cohort) and 15 patients (local cohort) due to an absence of indication for EVT or allergy to contrast product. For the patients in the local cohort without a CTA at presentation: six patients had a recent vascular imaging of the neck or a carotid doppler during the hospitalization, four patients died before performing vascular imaging and five patients did not receive vascular imaging since they were not considered candidates for vascular surgery and another stroke mechanism seemed more likely.
** Because of invasive procedure, bleeding, recent stroke.
*** Atherosclerosis, dissection, endovascular intervention, small-vessel disease, prothrombotic state, dural fistula, zoster vasculitis.
Baseline characteristics of the local cohort were similar to the whole cohort, except for fewer LVO and lower baseline NIHSS (Table 1). Transthoracic echocardiography (TTE) was performed in 38 (59%) patients. Of these, left atrial volumes were found to have severely enlarged in 15 and moderately enlarged in 3 based on Lang’s criteria. Reference Lang, Badano and Mor-Avi9 Among surviving patients at discharge (n = 44), OAC management was highly heterogeneous (Figure 1). The outcome at 3 months was available for 57 (88%) patients, with median (IQR) 90-day mRS 4 [2–6]. Nineteen (33.3%) patients had a favorable outcome (mRS 0–2) and 21 (32.3%) patients died.
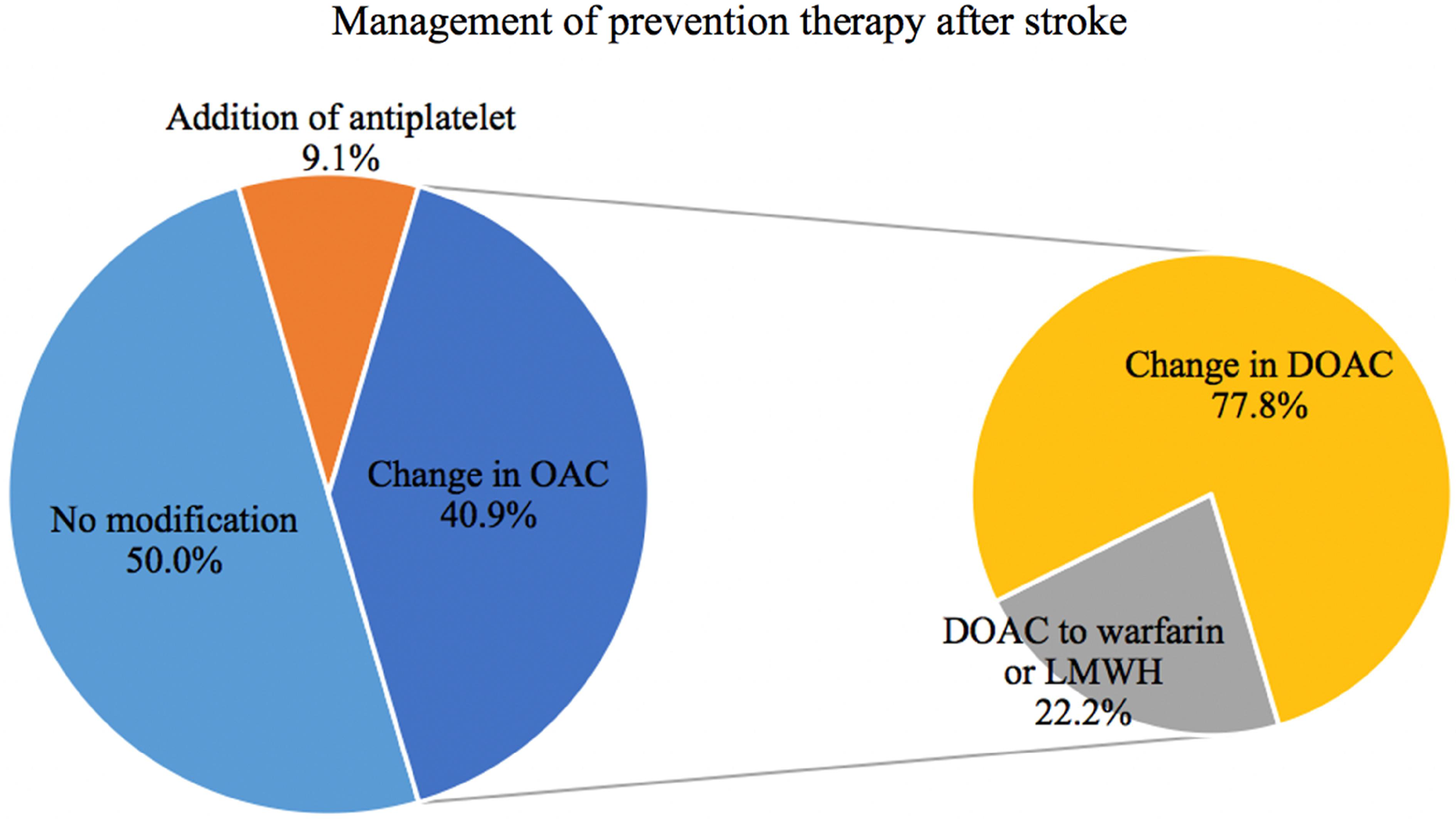
Figure 1: Management of secondary stroke treatment in patients with an ischemic stroke despite current anticoagulation in the local cohort. (21 patients died before restarting anticoagulation treatment, results shown for 44 patients total). LMWH = low molecular weight heparin
In our study, modifiable and preventable causes of ischemic stroke were identified in 30% of patients; results that are in line with previous findings, Reference Polymeris, Meinel and Oehler10 albeit in contrast to other studies suggesting that patients on DOACs typically achieve high rates of adherence. Reference Polymeris, Traenka and Hert11 Off-label inappropriate under-dosing of DOAC remains an important cause of ischemic stroke despite DOAC therapy, Reference Paciaroni, Agnelli and Caso12 with rates reaching 17% in our study.
Periprocedural management of anticoagulation in patients undergoing invasive procedures is another major cause of ischemic stroke despite OAC use. In our study, 14.5% of patients presented with an ischemic stroke due to OAC interruption, of which 80% were on account of an invasive procedure. Interrupting anticoagulation for an invasive procedure transiently increases the risk of thromboembolism. Reference Kearon and Hirsh13 Despite published literature and clinical practice recommendations, Reference Baron, Kamath and McBane14 periprocedural anticoagulation managements remains heterogeneous, with inappropriately prolonged OAC interruption and subsequent embolic risk. Reference Kurlander, Barnes and Anderson15 Details regarding OAC interruption were not systematically captured and as a result, we were unable to decipher whether OAC management followed guideline recommendations. Evidently, systemic capture of these data is necessary to optimize periprocedural antithrombotic management.
In previous studies, 30% of patients with ischemic stroke despite OAC had a non-cardio-embolic cause of stroke, Reference Paciaroni, Agnelli and Caso12 as supported by our local cohort, in which a competing cause of stroke was found in approximately 20% of patients. Whether cardiovascular risk factor management was optimal in our patients is not known, but the presence of other cardiovascular risk factors in patients taking OAC for AF increases the risk of recurrent stroke. Reference Seiffge, De Marchis and Koga4,Reference Paciaroni, Agnelli and Caso12
Of patients who underwent TTE, severe left atrial enlargement was found in a significant proportion of patients. Indeed, several studies suggest that left atrial enlargement severity, a marker of atrial cardiopathy, is associated with increased stroke risk, particularly in those with ischemic stroke despite OAC. Reference Stöllberger, Chnupa and Kronik16–Reference Diener, Easton and Granger19 Left appendage morphology was not described in TTE reports, although this has been shown to be associated with the risk of stroke. Reference Di Biase, Santangeli and Anselmino20 It is currently unknown whether the presence of left atrial enlargement or left appendage morphology should modify anticoagulation regimens or periprocedural anticoagulation interruption in patients with AF.
Regarding post-stroke antithrombotic management, our findings show that practice patterns were heterogeneous, reflecting the lack of evidence to guide clinicians in this context. Reference Gladstone, Lindsay and Douketis8 In 50% of patients, physicians chose to continue prior anticoagulation, while in the other half, the anticoagulant agent was either changed or complemented with the addition of an antiplatelet agent. A recent survey found similar practice patterns. Reference Salehi Omran, Parikh and Zambrano Espinoza21 Nevertheless, changing the type of anticoagulant may not help to reduce the risk of future ischemic strokes. Reference Seiffge, De Marchis and Koga4
Our study has several limitations. Although all patient data was collected prospectively as part of clinical care, as a retrospective study, information regarding, stroke workup and follow-up data were not available after CSC discharge for the majority of our population given PSC repatriation protocols. Furthermore, information was not available to discern whether periprocedural management and OAC interruption was appropriate and guideline-based in most patients due to the retrospective nature of the analyses. Lastly, since this is a retrospective observational study with relatively small sample size in a single CSC, we were limited in detecting significance differences in our results, and our findings may not be generalizable. Furthermore, the possibility of selection bias exists, particularly regarding more severe strokes seen at our institution, potentially skewing the results. Finally, the relatively short follow-up period limits the ability to assess the risk of recurrent stroke in this high-risk population.
Overall, one-third of stroke despite therapeutic OAC was identified to be secondary to preventable causes such as inappropriate OAC dosing and a lack of treatment compliance. Patient and physician education regarding the importance of adequate OAC dosing, treatment compliance and guideline-based periprocedural OAC management should be emphasized to reduce ischemic stroke risk while prospective studies are warranted to improve secondary stroke prevention in this patient population.
Author contributions
Study conception and design, data collection, statistical analysis, manuscript writing: MCD, CD, LG.
Gathering and inclusion of patients in the prospective registry, manuscript revision: ND, YD, GJ, CO, CS, AYP, LG.
Cardiology expertise and manuscript revision: GR.
Funding
An educational bursary was provided by Servier Inc. for a clinical trainee (CD) involved in the study.
Competing interests
AYP reports research grants from CIHR and Fondation Brain Canada; compensation from Roche for other services (speaker’s honoraria); grants from Heart and Stroke Foundation of Canada; and grants from Stryker. GR reports speaker’s honoraria from Canadian Cardiovascular Society, Société des sciences vasculaires du Québec, Fédération des omnipraticiens du Québec and Congrès cardiovascular du CHUM. LG has received funding from the Heart and Stroke Foundation of Canada (project no. G-23-0034223) as well as advisory board or speaker honoria from Astrazeneca, Bayer, BMS Pfizer, Servier. The remaining authors have no competing interest.




