Introduction
The prevalence of epilepsy in children in Canada is approximately 3/1000.Reference Gilmour, Ramage-Morin and Wong1 Up to 30% of children will insufficiently respond to at least two first-line medications and have drug-resistant epilepsy.Reference Moshé, Perucca and Ryvlin2 In these patients, alternative therapies are often considered, including the ketogenic diet,Reference Rogovik and Goldman3 vagus nerve stimulation,Reference Hatton, McLarney, Pittman and Fahy4 epilepsy surgery,Reference Dwivedi, Ramanujam and Chandra5 and cannabis.Reference Hausman-Kedem, Menascu and Kramer6
There is emerging evidence that some ingredients of cannabis (e.g., cannabidiol (CBD) and tetrahydrocannabinol (THC)) may have therapeutic effects.Reference Hausman-Kedem, Menascu and Kramer6 CBD and THC have distinct properties that make them useful in different therapeutic settings. THC has been shown to improve mood, increase appetite, and act as an analgesic.7 CBD has neuroprotective, antiepileptic, antipsychotic, analgesic, anti-inflammatory, and anti-asthmatic properties.7 Unlike THC, CBD does not cause euphoria,Reference Hausman-Kedem, Menascu and Kramer6 making it a potentially better choice for patients. In combination products, higher concentrations of THC can cause more psychoactive effects.Reference Koppel, Brust and Fife8
There are several randomized control trials (RCTs) illustrating beneficial effects of cannabinoid treatment in children with epilepsy. In a trial of 20 children with Dravet syndrome given a mix of CBD and THC, 14 patients experienced ≥50% reduction in seizure count.Reference McCoy, Wang and Zak9 In a study of 120 patients with Dravet syndrome, children using CBD experienced a median seizure frequency reduction of 39% compared to a median of 13% in the placebo group.Reference Devinsky, Cross and Laux10 In a study of 171 children with Lennox–Gastaut syndrome, patients taking CBD experienced a median reduction in seizure frequency by 44%, compared to a median of 22% in the placebo group.Reference Thiele, Marsh and French11 In all three studies, the majority of children taking cannabinoids experienced only minor side effects, the most common ones being fatigue, gastrointestinal disturbances, and decreased appetite.Reference McCoy, Wang and Zak9–Reference Thiele, Marsh and French11 These results have led to the recent Food and Drug Administration (FDA) approval of CBD as a therapy for Dravet syndrome and Lennox–Gastaut syndrome in the United States.Reference Billakota, Devinsky and Marsh12
Cannabinoids have also been reported to improve the quality of life of children with epilepsy. Pediatric epilepsy patients treated with CBD have demonstrated improvement in cognition, social interactions, and overall quality of life, in addition to a reduction in seizures.Reference Porter and Jacobson13,Reference Rosenberg, Louik and Conway14 With the exception of one study, most of the research has demonstrated beneficial effects of CBD alone. However, in Canada, CBD is currently only available in combination with THC.
Canadian healthcare practitioners can legally authorize medical cannabis for the treatment of epilepsy in patients of all ages. However, there are currently no guidelines with regard to its use in children with epilepsy, and little is known about neurologists’ opinions and hesitations toward its use in children.
Methods
A 21-item questionnaire was created using the secure, web-based application REDCap,Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde15 hosted by the Children’s Hospital of Eastern Ontario (CHEO). Seventeen questions were related to the physician’s style of practice and opinions toward cannabis, and the remaining four were demographic questions (see Supplementary Material). All questions were mandatory, with the exception of demographic questions.
The questionnaire was distributed via email to 148 pediatric neurologists. All pediatric neurologists in Canada were eligible to complete this survey, and there were no exclusion criteria. Contact information of neurologists was obtained based on the methods of a similar study.Reference Buttle, Sell and Lemyre16 A list of registered pediatric neurologists from the licensing body was not available, so an initial Google search of all hospitals in Canada offering pediatric neurology services was conducted. Physicians’ email addresses were obtained from each hospital’s neurology department website or local medical school website. To ensure a comprehensive list and accurate contact details, hospital and medical school administrative assistants were contacted to provide verification. In the event that the physician’s email was not publicly accessible, the administrative assistant was asked to provide it if the physician consented. Pediatric neurologists working in the community were also contacted. Colleagues in different provinces were asked to provide the names of community neurologists working in their area. Email addresses of these physicians were similarly obtained from their affiliated hospital or private clinic website.
Participants were randomly assigned a participant number. The number was dissociated from their email address to ensure anonymity. Participants were given 4 weeks to complete the questionnaire once it was received, and a reminder was sent 2 weeks before the closing date. Consent was implied upon completion of the survey. Once completed, participants were given the option to enter their email address into a lottery system to win a $100 gift card. This page was created as a separate online survey, which was disconnected from the participant’s previous responses. The study design received approval from the CHEO Research Ethics Board.
Study data were collected and managed using REDCap electronic data capture tool.Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde15 Data analysis was performed using the R statistical programming language.
Suspected outliers were detected and adjusted. Three respondents reported that over 1500 children with epilepsy were currently in their practice. From inspecting a histogram of the variable’s distribution, these values appeared to be outliers. It is clinically unlikely that such a large number of epilepsy patients would be under the care of one neurologist at a given point in time. Values were adjusted assuming these cases were a data entry error (e.g., two values of 2500 were adjusted to 250, and one value of 1600 was adjusted to 160). Reassuringly, when these patients were excluded from the study, the qualitative findings were unchanged.
Results
Participant Demographics
Fifty-six of the 148 contacted pediatric neurologists completed the survey, achieving a response rate of 38%. Forty-four of the respondents reported practicing in a hospital, five worked in a private practice, and seven did not provide this information. Neurologists who provided responses were from all provinces except New Brunswick, with the majority from Ontario (Figure 1). Eight participants did not specify which province they worked in. Thirty neurologists had a fellowship in epileptology or equivalent training. The participants practiced for a range of 0–45 years, with an average of 13 years and a median of 10 years. The average number of pediatric neurologists to have worked at a respondent’s institution was 7, the median was 6, and the range was 1–30.

Figure 1: Geographic distribution of neurologist locations. Respondents were from all provinces except New Brunswick; however, eight respondents did not specify their province of origin and are, therefore, not included in the figure.
Cannabis Treatment Pattern in Children
All 56 responding neurologists treated children with epilepsy. The number of pediatric epilepsy patients they currently followed ranged from 10 to 800, with a median of 200. The median number of pediatric epilepsy patients being treated with cannabis was 5 per neurologist, with 3 and 12 patients in the 25th and 75th percentiles. The maximum was 80 pediatric epilepsy patients treated with cannabis by one neurologist. In total, 668 pediatric epilepsy patients were reportedly being treated with cannabis.
Perceived Cannabis Treatment Outcomes
When asked what percentage of children on cannabis experienced a reduction in seizure frequency, eight respondents reported that none of their patients had a reduction. The majority of respondents stated that no more than one-third of their patients benefited from cannabis; 11 reported that up to two-thirds experienced a reduction in seizure frequency; and three reported that almost all patients benefited (Table 1).
Table 1: Summary of pediatric neurologists’ perceived outcomes in patients who received cannabis treatment for epilepsy. Only those respondents, 51 in total, who reported that at least one of their patients were being treated with cannabis responded to these questions

With regard to patients suffering side effects, only three neurologists reported none. The majority reported side effects in up to one-third of their patients; 11 reported side effects in up to two-thirds of patients; and six reported that almost all patients experienced side effects (Table 1).
Pediatric Neurologist Attitudes Toward Cannabis
All surveyed neurologists reported at least one hesitation (Figure 2). The most common hesitations were poor evidence, poor quality control, and cost. Other hesitations included side effects, using it to treat a non–drug-resistant epilepsy, difficulty authorizing CBD or finding an outside authorizer, young age, medicolegal concerns, and risk of addiction. Thirty neurologists (54%) believed there was sufficient evidence to treat childhood epilepsy with cannabis; 23 (41%) stated there was insufficient evidence; and three (5%) were unsure. Only 13 neurologists (23%) felt confident in the quality control of medical cannabis in Canada; 30 (54%) did not feel confident; and 13 (23%) were unsure.
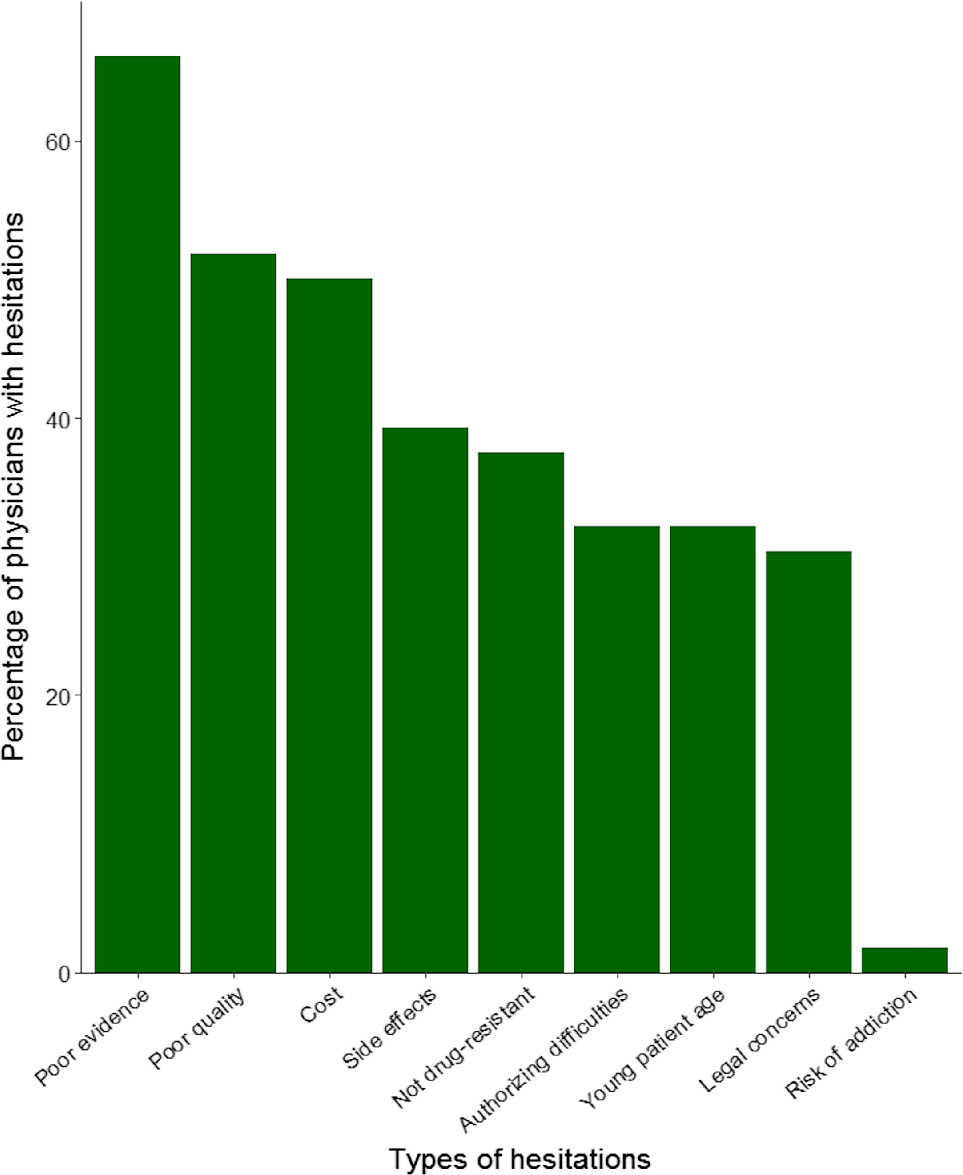
Figure 2: Percentage of physicians who held each hesitation when considering cannabis treatment for children with epilepsy. All 56 neurologists responded to this question and reported at least one hesitation. The shortest bar represents one respondent.
Pediatric Neurologist Authorization Patterns of Cannabis
The decision to prescribe cannabis was influenced by patient age. Thirty-three (83%) physicians who treat children with cannabis would consider starting treatment at the age of 2 years (Figure 3). For children under 2 years, only a minority of physicians would consider cannabis treatment (two for newborns, six for 6-month-olds, and 14 for 1-year-olds).

Figure 3: Distribution of age at which pediatric neurologists considered treating their epilepsy patients with cannabis. A total of 40 physicians responded to this question, with the shortest bar size representing one respondent (2.5%). Only one physician reported withholding cannabinoid treatment until patients turned 18 years.
Cannabis was authorized by roughly two-thirds of neurologists, either directly or through a referral. The remaining third did not authorize or refer their patients to anyone. Seven of those who did not authorize reported they plan to do so in the near future. Twenty-one neurologists made referrals to community practitioners, most often general practitioners. Two neurologists referred their patients to hospital-based practitioners (Figure 4). Following a referral, one neurologist reported modifying their patient’s cannabinoid dosage; four stated that their decision to modify varied between patients; and 17 did not modify cannabinoid dosage but rather left dose adjustments up to the cannabis authorizer.

Figure 4: Clinical pathway related to treating pediatric epilepsy patients with cannabinoids for a cohort of Canadian pediatric neurologists. The count of responding neurologists is provided for each pathway, with conditional probabilities.
Wait Times
It took an average of 2 months for patients to receive an appointment once they were referred for authorization. The longest wait time reported by neurologists based on their patients’ experiences was 6 months.
Indications
Cannabinoids were never considered as a first-line therapy. Most physicians only used cannabinoids after an average of three failed anticonvulsants. The spread of the middle 50% of data is between two and four failed anticonvulsant attempts (i.e., the interquartile range was 2). All respondents reported that their patients were taking cannabis as an anticonvulsant, and five reported that their patients took it as-needed for seizure abortion. Dravet syndrome was the most common diagnosis that neurologists considered authorizing cannabis for, followed by Lennox–Gastaut syndrome. Sixteen neurologists considered it for other types of epilepsy (Table 2).
Table 2: Summary of indications for authorization and type of cannabis used. Only those respondents, 41 in total, who reported authorizing or referring their patients responded to these questions

* The type of cannabinoid a patient was taking was based on the perception of neurologists. The cannabinoid type reported by some neurologists is not available in Canada; therefore, we assumed that some neurologists were misinformed.
Cannabinoid Type
Nearly half of the neurologists reported that their patients were taking cannabis in a CBD-to-THC ratio of 20:1. Just over half reported that their patients were taking CBD alone, and very few reported that their patients were taking THC alone. Some neurologists were unsure which form of cannabis their patients were taking. The majority of neurologists would prefer CBD alone if it were readily available in Canada; some preferred CBD in combination with THC; and some had no preference. None of the respondents preferred THC alone (Table 2).
Discussion
With many gaps in evidence and high patient-driven demand for cannabis therapy in Canada, our survey provides information from the ‘wisdom of the crowd’ until further evidence is available.
We recognize the limitation that we do not have a population-based sample; however, we believe our data could perhaps be used as a very rough estimate of the current proportion of Canadian children who might be receiving cannabis for epilepsy. In 2018, an estimated 7.2 million children were living in Canada17 and epilepsy prevalence was 3/1000,Reference Gilmour, Ramage-Morin and Wong1 meaning approximately 21,000 children had epilepsy. Conservatively, we estimated that at least 1000 of these children were treated with cannabinoids during the summer of 2018. This estimate was based on the total of 668 patients undergoing treatment from the neurologists who responded to this survey, with the assumption that the two-thirds of neurologists who did not respond may be less likely to treat with cannabis. Therefore, we estimated that at least approximately 5% of all Canadian children with epilepsy were being treated with cannabis during this time. This number is relatively low, considering that 30% of epilepsy patients are resistant to first-line, conventional antiepileptic medications.Reference Moshé, Perucca and Ryvlin2 This number is also lower than what has been discussed in studies from other countries. In an Australian survey, out of 389 children with epilepsy, 13% were given cannabis to treat their epilepsy.Reference Suraey, Todd and Bowen18
Canadian pediatric neurologists have many hesitations about treating children with cannabis. Two-thirds felt that there was poor evidence to support the use of cannabis in children; however, over 90% of these neurologists at some point either authorized cannabis to their patients or provided a referral. We speculate that one of the reasons for this discrepancy is a high patient-driven demand for cannabis therapy. Though this statement cannot be confirmed from our data, in a 2017 Canada-wide survey, 38% of general pediatricians reported being asked by a patient or their parent to authorize cannabis.Reference Belanger, Grant and Cote19 Despite this demand, our results indicate that some patients did not have access to cannabis, as 29% of neurologists did not authorize it or refer their patients to another physician for authorization. Based on a median of 200 epilepsy patients followed by each neurologist, we estimated that up to 3200 patients were not provided the option to receive cannabis as an anticonvulsant treatment. Taking into consideration that around one-third of these patients will not be adequately managed with anticonvulsants, more than 1000 pediatric epilepsy patients who may benefit from cannabis therapy were not provided the option.
The percentage of patients who benefited from cannabis therapy, according to the neurologists in our study, differed from what has been described in the literature. The majority of neurologists in our study reported that only up to 32% of patients experienced a reduction in seizure frequency. However, the lowest reported percentage in RCTs of patients benefiting from cannabis as an anticonvulsant was 40%.Reference Devinsky, Cross and Laux10,Reference Thiele, Marsh and French11 One trial illustrated a benefit in as high as 70% of patients.Reference McCoy, Wang and Zak9 Similarly, the percentage of patients who experienced side effects differed between our results and the literature. The results of three RCTs were that side effects occurred in 95%,Reference McCoy, Wang and Zak9 93%,Reference Devinsky, Cross and Laux10 and 86%Reference Thiele, Marsh and French11 of patients; however, the majority of neurologists in our study reported only up to 32% of their patients experienced side effects. One explanation for these differences may be that patients in clinical practice are using lower doses of cannabinoids than those in RCTs, as in our clinical experience, patients would hesitate to use higher doses of cannabis due to its cost. Similarly, most RCTs were conducted in countries other than Canada, and therefore different cannabis products may have been used. Furthermore, published trials only included patients with DravetReference McCoy, Wang and Zak9,Reference Devinsky, Cross and Laux10 and Lennox–Gastaut syndrome,Reference Thiele, Marsh and French11 whereas patients in our study may have had other types of epilepsy.
We found variability in patient age at which neurologists considered cannabis treatment (Figure 3). Just under half of the neurologists felt confident initiating cannabis therapy for patients under 2 years of age, possibly secondary to the fact that there have only been RCTs evaluating cannabis efficacy starting at ≥2 years of age.Reference McCoy, Wang and Zak9–Reference Thiele, Marsh and French11,Reference Devinsky, Patel and Cross20
Similarly, there is variability in diagnostic indications for cannabis treatment. For pediatric Dravet syndrome and Lennox–Gastaut syndrome, there are RCTs supporting cannabis use.Reference Devinsky, Cross and Laux10,Reference Thiele, Marsh and French11 This may explain why 64% of surveyed neurologists would consider cannabis for these epilepsy syndromes. However, a number of neurologists also considered cannabis for other types of epilepsy. There is currently no evidence for cannabis therapy in pediatric epilepsy other than Dravet or Lennox–Gastaut syndrome, though there is some research in adult populations. Adults with idiopathic generalized epilepsy undergoing treatment with CBD in an RCT experienced a greater reduction in seizure frequency compared to those taking a placebo.Reference Cunha, Carlini and Pereira21 In case studies, adults with focal epilepsy undergoing treatment with CBD experienced reductions in seizure frequency, some of which also experienced an exacerbation in seizure frequency with cannabis cessation.Reference Hegde, Santos-Sanchez and Hess22,Reference Mortati, Dworetzky and Devinsky23
Our results suggest that potential miscommunications exist between patients, cannabis authorizers, and neurologists regarding cannabis intake. Many neurologists were under the impression that their patients were taking CBD without THC, though, to the best of our knowledge, this option is not readily available in Canada. It is possible that neurologists were not accurately informed, since the majority did not follow up to modify the cannabinoid dosage after referring their patients. Furthermore, four neurologists explicitly stated that they were unsure which form of cannabis their patients were taking.
Five neurologists reported that their patients were taking cannabis as needed for seizure abortion. Currently, there is no evidence supporting cannabis for this indication, as all RCTs have only tested cannabis as an anticonvulsant.Reference McCoy, Wang and Zak9–Reference Thiele, Marsh and French11,Reference Devinsky, Patel and Cross20 Since this question was based on physicians’ observations of their patients’ use of cannabis and not on how the physician authorized it, it is possible that these patients were either using this product against their physician’s recommendations, were not properly educated about indications of use of cannabis in epilepsy, or were using it as an experimental, non-evidence-based drug, with or without their physician’s approval.
The number of neurologists in our survey who were confident in the level of evidence for cannabis differed from other surveys. Compared to the 54% (30/54) in our study, 28% (48/171) of neurologists and epileptologists from North America and Europe felt there was sufficient evidence to support the use of cannabis in the treatment of epilepsy.Reference Mathern, Beninsig and Nehlig24 Though this percentage is lower than that of our study, the other survey did not specify whether the treatment was for children or adults, nor did it include the phrase “for treatment of select patients with epilepsy,” as our questionnaire did. However, 48% of European and North American neurologists support cannabis use in patients specifically with refractory epilepsy, which is more comparable to our results. Of note, that study also reported much higher support rates for therapeutic cannabis use in epilepsy among allied health professionals and general practitioners (70%, 42/59).Reference Mathern, Beninsig and Nehlig24 Similarly, in an Australian survey, 70% (441/627) of general practitioners supported the use of cannabis for epilepsy treatment.Reference Karanges, Suraev and Elias25
When generalizing these results to all pediatric neurologists in Canada, few limitations of this study should be noted. Though we used our best efforts to contact all pediatric neurologists in Canada, we could not get a comprehensive list from the licensing body and, instead, had to conduct a Google search. As a result, those neurologists whose names were not publicly available or provided by clinic administrative assistants may not have been contacted and, therefore, were not represented in the study. This survey achieved a response rate of 38%, with the largest response received from physicians in hospitals in Ontario. Neurologists who responded to the survey may have more polarized opinions toward cannabis than the general population of pediatric neurologists, but there is no way to assess this possible source of bias. There were three outliers that had to be adjusted, as it was unlikely for one neurologist to be following over 1000 epilepsy patients at one time. However, this adjustment did not alter the qualitative findings and, therefore, did not impact the results of the study. Some children may have been seen by more than one neurologist and, therefore, were possibly represented more than once in the survey, potentially skewing the results. Our attempt to estimate the proportion of Canadian children undergoing treatment with cannabis for epilepsy could be misleading due to the sample size and possible bias from the respondents who completed this survey. Future work using health administrative studies is required to more rigorously address this. Reassuringly, this survey received a similar response rate to our team’s previous research survey,Reference Buttle, Sell and Lemyre16 which is in the expected range of healthcare research response rates.Reference Phillips, Friedman and Utrankar26 Though this survey might provide useful information for neurologists, it cannot be used as a surrogate for guidelines when treating children with epilepsy.
In conclusion, this study describes the attitudes and clinical practice of Canadian neurologists, many of whom were from Ontario, regarding cannabis therapy for pediatric epilepsy patients prior to the legalization of cannabis for recreational use. Keeping in mind the limitations of the survey, we conservatively estimated that at least 1000 pediatric epilepsy patients were being treated with cannabinoids during the summer of 2018, which equates to approximately 5% of all children with epilepsy in Canada, and every sixth pediatric patient with drug-resistant epilepsy. Although the use of cannabis is widespread, all surveyed neurologists had hesitations about cannabis therapy, including poor evidence, poor quality control, and high cost. The high patient-driven demand for cannabis therapy, combined with neurologists’ hesitations and many research gaps, makes the efficacy of cannabis a key topic for future research. While awaiting new evidence and subsequent clinical practice guidelines, our survey provides information to help neurologists contextualize their clinical decisions.
Acknowledgments
We thank the pediatric neurologists in Canada who responded to the questionnaire. We also thank the pediatric neurologists at the Children’s Hospital of Eastern Ontario for their feedback when developing the questionnaire.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
All listed authors meet authorship criteria. The specific contributions made by each author are described. SMDG: data acquisition, analysis and interpretation of data, drafting the manuscript, and approval of the version of the manuscript to be published. RW: design of the study, analysis and interpretation of data, critically revising the manuscript for intellectual content, and approval of the version of the manuscript to be published. DP: conception and design of the study, critically revising the manuscript for intellectual content, and approval of the version of the manuscript to be published.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.50.








