Autoimmune encephalitis is becoming an increasingly recognized treatable entity in patients with refractory seizures, behavioural changes, memory or cognitive impairment, or autonomic dysfunction. The clinical, biochemical, electrographic, and imaging characteristics of different autoantibody mediated central nervous system (CNS) disorders are a focus of interest since early recognition and treatment may avoid inflammatory CNS damage.Reference Irani, Stagg and Schott1, Reference Hébert, Day, Steriade, Wennberg and Tang-Wei2 One group of autoantibodies directed toward the leucine-rich, glioma-inactivated-1 (LGI1) protein within the voltage-gated potassium channel (VGKC) complex is associated with a classic clinical phenotype of hyponatremia and faciobrachial dystonic seizures (FBDSs). These seizures are characterized by short-duration dystonic movements of the face and ipsilateral arm that usually do not cause altered consciousness. On magnetic resonance imaging (MRI), the typical finding in anti-LGI1 limbic encephalitis is T2 hyperintensity within the mesial temporal lobes and basal ganglia.Reference Irani, Michell and Lang3, Reference Heine, Prüss, Bartsch, Ploner, Paul and Finke4 Interestingly, there have been reports of patients with FBDS who do not have presence of anti-LGI1 autoantibodies but are found to have electroclinical insular seizures with insular hyperintensity on MRI.Reference Patira, Khatri, Gutierrez and Zubkov5 We present a patient with FBDS and an isolated insular lesion that improved with immune suppression, highlighting the need for early recognition and treatment of potential autoimmune encephalitis despite atypical imaging findings.
A healthy 53-year-old right-handed man presented to the emergency department (ED) with multiple falls and posturing of his right arm and face with associated hypersalivation without loss of awareness. His wife noted occasional twitching of his right arm and face, which began to involve his leg and increased in frequency to 10 times per hour, causing him to fall. Although the patient stated he did not notice the movements, he acknowledged overwhelming feelings of anxiety after a spell. These were consistent with FBDS. Examination was significant for upper motor neuron distribution right facial weakness and several dystonic movements while ambulating in the ED, one of which resulted in a fall. The movements were suppressible with distraction and became more pronounced when discussing distressing matters.
Blood work revealed hyponatremia at 127 mmol/L, and MRI showed an isolated left insular T2 hyperintensity (Figure 1A). A routine electroencephalogram (EEG) was unremarkable; however, subsequent 24-hour continuous video EEG captured 15–25 FBDS per hour with retained awareness and on rare occasions associated with 15–20 seconds of rhythmic theta activity peri-ictally, maximal over left fronto-temporal electrodes (Figure 2A, Supplement 1). In a 90-minute epoch of recording, 6 out of 32 events sampled (19%) had the rhythmic electrographic theta signature present within 30 seconds of the FBDs; 4 with a mean onset of 19 seconds after the FBDS ended, 1 without a clear clinical association and 1 occurring 8 seconds prior to the FBDS (Figure 2A). Infraslow activity (ISA) over the left frontocentrotemporal derivations was observed leading into 75% of the FBDS (Figure 2B). Interestingly, none of the events associated with the theta activity described above had ISA leading into the ictus. Initial cerebrospinal fluid (CSF) analysis showed elevated protein at 0.87 g/L, normal glucose, normal range leukocytes at 2 × 106 cells/L and was sent for onconeural paraneoplastic antibodies, as well as anti-VGKC antibody panel, consisting of anti-LGI1 and anti-contactin-associated protein 2, to Mitogen Advanced Diagnostics Laboratory at the University of Calgary. The leading differential diagnosis was anti-LGI1 limbic encephalitis, and intravenous immunoglobulin was initiated as well as levetiracetam for seizure control.
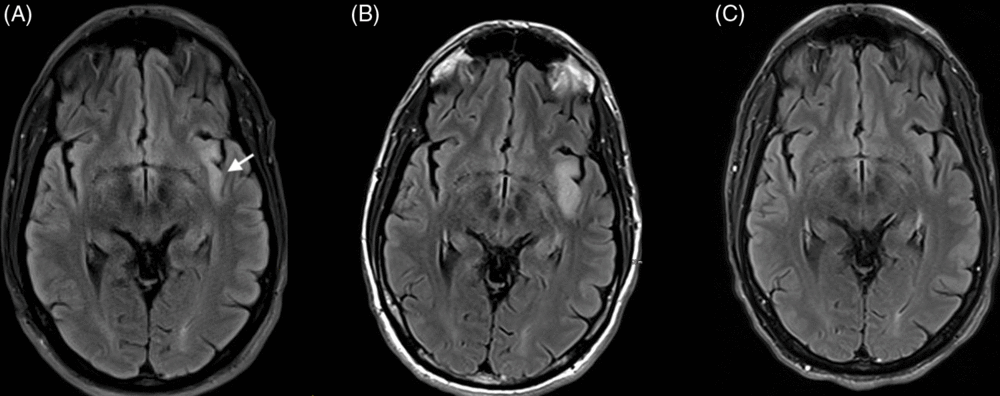
Figure 1: Axial T2 FLAIR MRI at baseline, day 10, and day 41. (A) Baseline MRI shows the left insular hyperintensity (arrow). (B) Day 10 shows expansion of lesion. (C) Day 41 shows resolution.
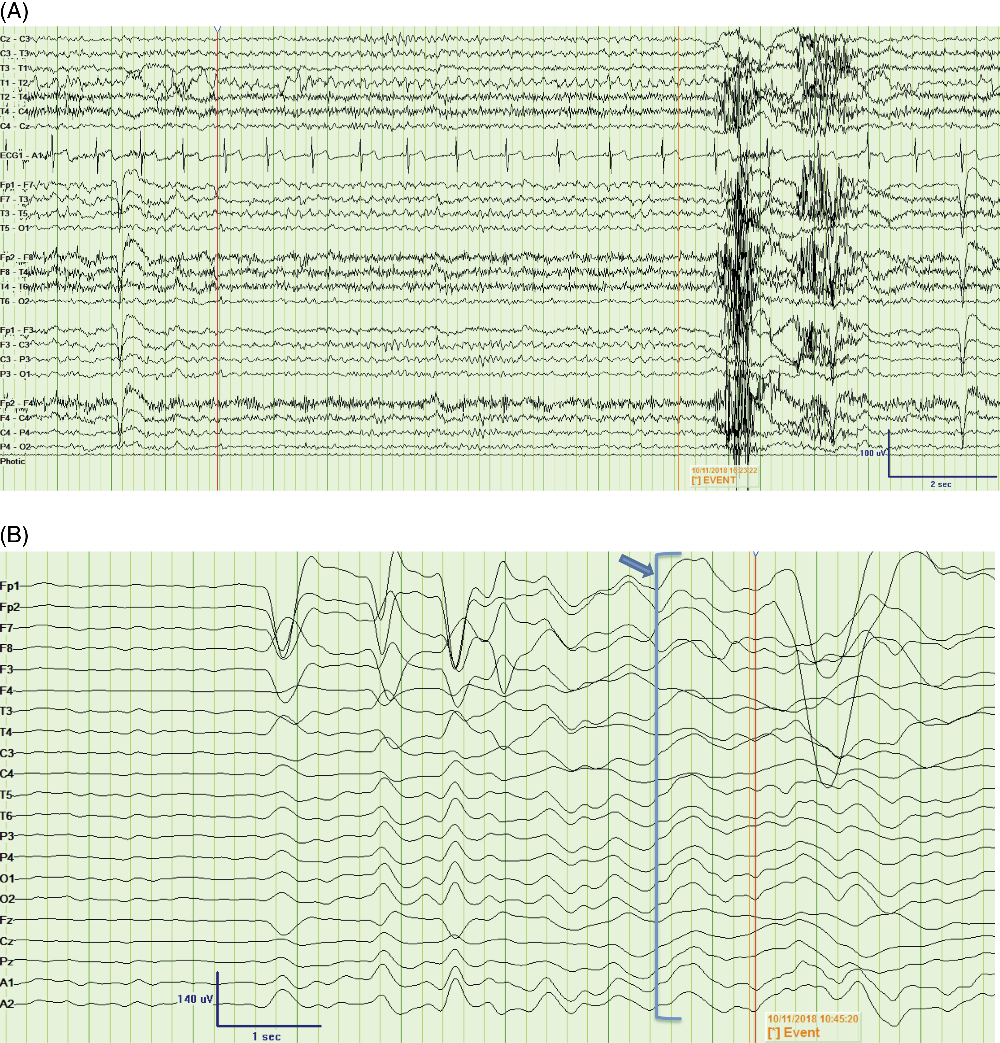
Figure 2: Peri-ictal EEG recordings from two time points. (A) Bipolar montage showing an example of 5-Hz rhythmic theta activity over the left frontotemporal derivations (F3, F7, T3), leading into the FBDS (marked by “Event”) [LFF = 1 Hz, HFF = 70 Hz]; (B) Average montage showing infraslow activity (arrow and bracket) leading into the FBDS (marked by “Event”) by approximately 750 msec, observed predominantly over the left frontocentrotemporal derivations [Expanded time base, LFF = 0.05 Hz, HFF = 5 Hz]. FBDS = faciobrachial dystonic seizure; LFF = low-frequency filter; HFF = high-frequency filter.
The severity of FBDS reduced and the patient was able to ambulate without falling; however, the high frequency of movements and rapidly cycling symptoms of anxiety persisted. A repeat MRI was performed 10 days later and showed that the left insular T2 hyperintensity had increased from 35 mm × 9 mm to 35 mm × 14 mm in the axial plane, which is not typical for treated anti-LGI1 encephalitis. Differential diagnosis included low-grade glioma (Figure 1B). Subsequent MR spectroscopy revealed decreased N-acetylaspartate; however, there was no inversion of the choline to creatinine ratio, lipid or lactate peak, or diffusion restriction to suggest a neoplastic etiology. The lesion was thought to be secondary to peri-ictal changes since the FBDSs were ongoing. CSF antibody testing was negative for paraneoplastic antibodies though ultimately positive for anti-LGI1 autoantibodies.
The patient was subsequently treated with daily intravenous methylprednisolone sodium succinate 1000 mg for 3 days followed by a slow taper of oral prednisone. He had dramatic improvement of his movements and follow-up MRI one month later revealed resolution of the insular hyperintensity (Figure 1C). Repeat CSF and serum antibody testing after 2 months of treatment was negative and his cycling symptoms of anxiety slowly dissipated.
FBDSs are described in 30%–90% of patients with anti-LGI1 autoantibodies.Reference Irani, Michell and Lang3 Immune therapy for anti-LGI1 encephalitis is effective in reducing the frequency and recurrence of FBDS, as well as cognitive decline.Reference Irani, Stagg and Schott1, Reference Fantaneanu, Bhattacharyya, Milligan and Pennell6 Classic findings on MRI in anti-LGI1 encephalitis include mesial temporal lobe or bilateral basal ganglia T2 hyperintensities. Hyponatremia, potentially due to hypothalamic-mediated syndrome of inappropriate anti-diuretic hormone secretion, occurs in up to 60% of patients. Short-duration FBDS with normal EEG, disturbed consciousness or memory, autonomic disturbance or rapidly-cycling auras can also be present.Reference Heine, Prüss, Bartsch, Ploner, Paul and Finke4, Reference van Sonderen, Schreurs, Wirtz, Sillevis Smitt and Titulaer7 Two large case series of 23 and 196 patients with anti-LGI1 autoantibodies did not report isolated insular lesions.Reference Irani, Michell and Lang3, Reference Gadoth, Pittock and Dubey8 However, one report of paraneoplastic anti-LGI1 encephalitis had diffuse MRI T2 hyperintensity in the parieto-temporo-occipital lobes including the insular cortex.Reference Ahn, Kim, Kim, Lee, Ahn and Sung9 Insular seizures have been associated with FBDS, however, with negative anti-LGI1 antibodies.Reference Patira, Khatri, Gutierrez and Zubkov5 Typically, contralateral FBDS lasting over 30 seconds, hypersalivation, normal serum sodium, preserved consciousness, and EEG changes showing ictal epileptiform activity are indicative of focal insular seizures not caused by an autoimmune etiology. Our patient had some features of classic anti-LGI1 encephalitis as well as features of non-immune mediated FBDS from an insular lesion, such as the peri-ictal left frontotemporal theta pattern described above. Of interest, our findings confirm the presence of ISA at FBDS onset, previously described as a reliable electrographic signature of FBDS.Reference Wennberg, Steriade, Chen and Andrade10 Despite the non-classic MRI T2 hyperintensity of the left insular cortex and the EEG findings, our clinical suspicion for anti-LGI1 was high and we initiated high-dose immune therapy while the antibody results were pending, to good clinic effect. The fact that the antibodies for LGI1 returned positive affirmed that even patients with isolated insular lesions may have immune mediated disease and respond to immune suppression.
Regardless of atypical imaging findings or a non-classic presentation, early recognition of FBDS and its association with anti-LGI1 encephalitis is important to prevent diagnostic delay and initiate treatment to avoid secondary injury.
Disclosures
Dr. GB reports no disclosures. Dr. JCZ reports no disclosures. Dr. LDS reports no disclosures. Dr. NO reports no disclosures. Dr. TAF has received speaking honoraria from Sunovion, Eisai and Union Chimique Belge and a travel grant from Eisai. None are relevant to this case.
Statement of Authorship
GB: Designed and conceptualized study, analyzed the data, and drafted the manuscript for intellectual content.
JCZ: Interpreted the data, drafted, and revised the manuscript for intellectual content.
LDS and NO: Interpreted the data and revised the manuscript for intellectual content.
TAF: Designed and conceptualized study, analyzed the data, drafted, and revised the manuscript for intellectual content.
Supplementary Materials
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2019.247




