Introduction
The population in developed countries is growing older. In our own level 1 trauma center, the proportion of patients suffering from a traumatic brain injury (TBI) aged 70 years or older went from 18% in 2000 to 48.5% in 2012. Reference de Guise, LeBlanc and Dagher1 Older victims of trauma also often have significant co-morbid medical conditions and may be taking medications that can further complicate injury and resuscitation. Anticoagulants are frequently used in this age group for various indications. This class of medication poses a particular challenge to neurosurgeons in the setting of a TBI, as the clinician is caught between “a rock and a hard place”: anticoagulants may exacerbate intracranial hemorrhage, while their abstention may lead to thromboembolic complications.
Atrial fibrillation is the commonest indication for anticoagulation therapy. Indeed, atrial fibrillation is associated with an increased risk of embolic stroke, particularly when associated with other risk factors. Reference Gage, Waterman, Shannon, Boechler, Rich and Radford2 The CHADS2 scoring system gives points to specific risk factors. Warfarin can decrease the annual risk of stroke by half, from low to high CHADS2 scores. 3 Anticoagulants are also the mainstay of treatment for deep venous thrombosis (DVT), which, if not treated can lead to pulmonary embolism (PE) and post-phlebitic syndrome. It is also used to prevent DVT and PE in high-risk patients. Another common indication for anticoagulation is a metallic cardiac valve. A meta-analysis including 13,088 patients and studying 53,647 patient-years found that anticoagulation decreases the risk of valve thrombosis from 1.7% to 0.2%/year and decreases the risk of embolism from 4 to 1%/year. It also found that mitral valves have 5 times the risk of valve thrombosis and 1.5 times the risk of major embolism compared with aortic valves. Reference Cannegieter, Rosendaal and Briët4
Acute TBI is well known to be associated with abnormal coagulation parameters, Reference Laroche, Kutcher, Huang, Cohen and Manley5 especially within the first 24-48 hours. Expansion of intracerebral contusions and subdural hematomas, as well as extra-dural hematomas, is a common phenomenon in the first few hours to days after a TBI. Therefore, it is a universal practice to avoid anticoagulant medications in the first 24-48 hours following a TBI with intracranial bleed. Being on an anticoagulant also significantly increases the mortality of a traumatic intracranial bleed, Reference Cohen, Rinker and Wilberger6–Reference Mina, Knipfer, Park, Bair, Howells and Bendick7 while rapid reversal of anticoagulation therapy decreases the mortality. Reference Ivascu, Howells, Junn, Bair, Bendick and Janczyk8 After the acute period, the risk of intracranial hemorrhage expansion decreases sharply. However, a category of intracranial hemorrhage is prone to recurrent hemorrhages and expansion long after the trauma. Indeed, traumatic subdural hematoma (SDH) can enlarge and become symptomatic weeks and even months after a TBI, with a rate of significant spontaneous re-hemorrhage of 5. Reference Bajsarowicz, Prakash and Lamoureux9 Only one study looked specifically at the risk of SDH expansion with antithrombotic medication. Reference Pandya, Pattison, Karas and O’Mara10 Both anticoagulant and antiplatelet aggregation therapy were included, and it was restarted at a mean of 9 days post-admission, with a risk of significant intracranial hemorrhage expansion of 9%. Another study looked at the risk of rebleed with intracranial hemorrhages in previously anticoagulated patients, with some having resumed anticoagulation but it did not detail the specific risk of rebleeding for traumatic SDH while anticoagulated. However, SDH showed a significant risk of rebleed, anticoagulated or not (15%) and patients with intracranial hemorrhages also had a significant risk of rebleed when fully re-anticoagulated (20% vs 6%). Reference Hawryluk, Uastin, Furlan, Lee, Kelly and Fehlings11
The absence of guidelines is apparent when the neurosurgeons need to make a decision about patients suffering from a traumatic SDH and concomitantly requiring anticoagulation therapy. The goal of this study is to provide a better assessment of the actual risk of re-hemorrhage for patient suffering from traumatic SDH when anticoagulation therapy is initiated and balance it with the risk of withholding anticoagulation therapy.
Methods
Study population
All patients admitted to the Montreal General Hospital, an adult tertiary trauma center (level 1), with a diagnosis of traumatic SDH between January 2006 and January 2013 and prescribed anticoagulant therapy pre- or post-injury were considered for the study. First, the TBI program Database and the Trauma Registry Database were used to identify all patients admitted with a diagnosis of traumatic SDH. Second, a retrospective study of all charts (in-patient hospital charts and out-patient clinic notes) was performed to identify all those who were on anticoagulant therapy at the time of the injury and those who required anticoagulant therapy after the injury. Third, patients were excluded if (1) they died within 14 days of their admission due to the severity of the initial trauma, and if their death was unrelated to either post-trauma administration or withholding of anticoagulant therapy; (2) there was no indication for anticoagulation pre-injury and the indication post-injury came after a complete radiological resolution of the traumatic SDH; (3) the SDH was not traumatic; (4) the SDH was not acute; only patients presenting with an acute SDH were included in the study; (5) there was no SDH (incorrectly coded); or (6) charts were missing or incomplete after multiple attempts to locate them. The institutional Research Ethics Board reviewed and approved this study.
Patient management
All patients with a traumatic SDH were evaluated by a trauma team and by the neurosurgery service. Patients requiring immediate surgery, according to the Brain Trauma Foundation guidelines, were directed to the operating room. Therefore, patients with a symptomatic SDH greater than 10 mm and/or with an associated midline shift greater than 5 mm, patients with decreasing GCS score or showing signs of herniation or with increased intracranial pressure (ICP) in relation to the SDH were treated surgically. Reference Bullock, Chesnut and Ghajar12 According to the guidelines and the attending neurosurgeon’s clinical judgment, patients with small SDH, patients with asymptomatic SDH, or very elderly patients with minimal symptoms were treated conservatively. All patients treated conservatively were admitted to the intensive care unit under observation. All patients presenting with an INR above 1.4 received prothrombin complex to reverse the effect of warfarin. All patients with a GCS of 8 or less and an abnormal scan had an ICP monitor placed, according to the 2007 Brain Trauma Foundation guidelines. Reference Bratton, Chestnut and Ghajar13 Prophylactic anticoagulation was initiated in non-ambulatory patients 48 to 72 hours after the trauma, provided there was stability of the intracranial hemorrhagic lesions on two consecutive CT scans. Reference Dudley, Aziz and Bonnici14 Patients with non-surgical SDH upon admission with delayed clinical deterioration and with chronicization and volume expansion of the SDH were also treated surgically, by burrhole evacuation when amenable to it, or by craniotomy. Anticoagulation was resumed according to the following institutional protocol: patients with no residual SDH were simply started back on warfarin with bridging until a therapeutic INR was reached with low-molecular-weight heparin, except for cardiac valvular replacement patients; they were bridged on intravenous unfractionated heparin instead. Patients with a residual SDH were started on intravenous unfractionated heparin at a lower PTT target with no bolus injection. A CT head was done after 48 to 72 hours to verify if there was any new bleed. If no new bleed was detected, the patient was started on warfarin and switched to low molecular weight heparin as a bridging therapy, except for patients with cardiac valvular replacement. Those patients were kept on intravenous unfractionated heparin while warfarin was added until the INR was therapeutic.
CT scans were also performed 3-4 weeks after the traumatic event and every month afterwards as surveillance. Any new neurological symptom also prompted a CT scan.
Data collected
We first collected demographic data (age and gender) and injury-related data (mechanism of trauma, initial GCS score, injury severity score (ISS)). Reference Baker, O'Neill and Haddon15 We also collected data known to have influence on the rate of SDH re-hemorrhage (history of falls, alcohol abuse history). Reference Bajsarowicz, Prakash and Lamoureux9 The following imaging characteristics of the SDH on CT scan were also collected: SDH maximal thickness and location (convexity, parafalcine, tentorial, or posterior fossa). The specific anticoagulant agents as well as the medical indications for anticoagulation were recorded. The number of surgical evacuations and the type of evacuation (burrhole or craniotomy) done prior to starting/restarting anticoagulant therapy was noted.
We recorded how long the anticoagulant was held post-injury when patients were anticoagulated prior to their injury. We also looked at the CT scan characteristics of the SDH at the time of anticoagulation re-introduction and divided the patients into 2 groups: in the first group, the SDH had already completely resolved at the time of anticoagulation therapy resumption; in the second group, SDH remnant was still present. The quality of the remnant SDH could include acute re-hemorrhage, subacute, or chronic. These remnants were also subdivided into 2 groups, in order to see if the size of the remnant had an incidence on the risk of re-hemorrhage, with a “large” remnant being 5 mm or more in thickness and more than 3 cm in length and a “small” or “focal” remnant being less than 5 mm in thickness and 3 cm or less in length.
Outcome measures
The primary outcome measures were (1) the rate of adverse events related to the withholding of anticoagulation therapy (DVT, PE, stroke, transient ischemic attack, myocardial infarction, etc.…); (2) the number of SDH re-hemorrhages while anticoagulated. A SDH was considered to have re-hemorrhaged if there was on CT scan a new hyperdense component to the subdural collection. Fluid expansion alone was not considered a re-hemorrhage. The need for a surgical intervention due to the re-hemorrhage was also recorded.
Statistics
Descriptive statistics were reported for all variables as means and standard deviations for numerical variables, and percentages for categorical data. Chi-square test was used for comparisons.
Results
Patients sample and characteristics
The MGH TBI and Trauma Registry databases contained 1919 patients coded as “traumatic SDH” for the study time period. We found 137 patients who were on anticoagulant prior to the traumatic event. Forty-five patients were excluded because they died early from the severity if the injury (25), the SDH was not acute (15), or there was insufficient data (5). There were also three cases where anticoagulation therapy was not used before the traumatic SDH but was initiated post-trauma for DVT in two cases and DVT and PE in 1 case. A total of 95 patients were therefore included.
Seventy-one (75%) were male and 24 (25%) were female. The mean age was 74.4 with a standard deviation of 12 years and a range between 19 and 94. A majority of patients (83.2%) were aged 65 years and over. The mean GCS on admission was 13.4 (see table 1 for the breakdown of GCS). The great majority (82.1%) of patients suffered from a mild TBI (GCS between 13 and 15). The ISS was, however, 25.7 on average, ranging from 16 to 50. Table 2 details the mechanisms of injury. Ten patients (10.5%) were found to have a history of alcohol abuse, while 22 subjects in the sample (23.2%) had a history of recurrent falls.
Table 1: Glasgow Coma Scale score on admission
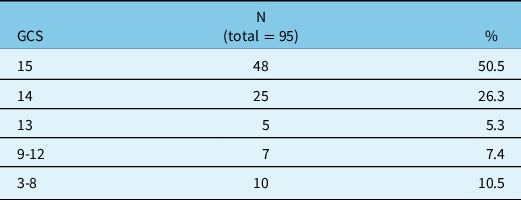
GCS = Glasgow Coma Scale score; N = number of subjects; % = percentage of the sample.
Table 2: Mechanism of injury
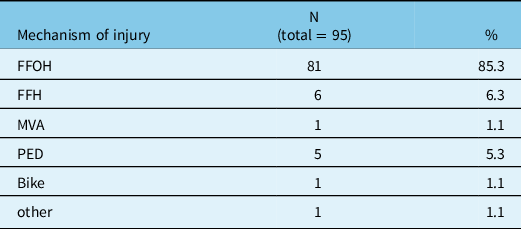
FFOH = fall from own height; FFH = fall from height; MVA = motor vehicle accident; N = number of subjects; PED = pedestrians hit by a car; % = percentage of the sample.
Imaging characteristics
The SDH on admission had a mean thickness of 11.5 mm, ranging from 1 to 43 mm, and a median of 10 mm. The majority (56.8%) measured 10 mm or less. The location of the SDH was as follows: 84 (88.4%) at the convexity, 7 (7.4%) tentorial, and 4 (4.2%) parafalcine.
Surgical treatment
Fifteen patients required an urgent evacuation of their SDH by craniotomy in the acute setting and another 3 required craniectomy along with the evacuation of the SDH. Fifteen patients underwent burrhole evacuation of their SDH in a delayed fashion once it became more chronic, prior to the institution or re-introduction of therapeutic anticoagulation.
Anticoagulation therapy
Table 3 summarizes the reasons for anticoagulation therapy prior to the injury. All but one patient used warfarin, with the remaining one using dabigatran. A significant number of patients (29 patients; 31.5%) also used antiplatelet aggregation agents in combination with warfarin (24 used acetylsalicylic acid, 3 used clopidogrel, and another 2 used acetylsalicylic acid and clopidogrel along with warfarin).
Table 3: Rationale for anticoagulation therapy
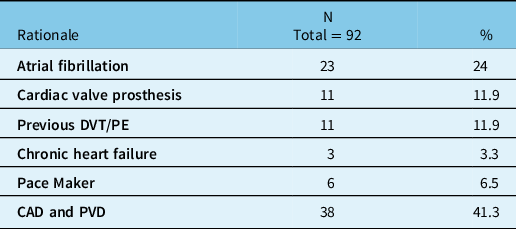
CAD = coronary artery disease; DVT = Deep venous thrombosis; N = number of subjects; PE = pulmonary embolism; PVD = peripheral vascular disease; % = percentage of the sample.
Timing of therapeutic anticoagulation re-introduction and complications
Anticoagulation therapy was held an average of 105.6 days and a median of 67 days (range 0-868 days). During the time therapy was held, there was one adverse event recorded (1.1%): a patient with previous mitral valve replacement was found to have an atrial clot on surveillance echocardiogram at 10 days following reversal and withdrawal of warfarin therapy. Therapeutic anticoagulation was reinitiated thereafter, and the patient did not develop embolic complication.
For the majority of patients (78 patients-82.1%), therapeutic anticoagulation was re-introduced when the SDH had completely disappeared at follow-up CT scan. For 17 patients (17.9%), therapeutic anticoagulation was restarted while the SDH had not completely resolved on CT scan. The residual was qualified as small in 9 patients and as large in 8 patients. In the group with a large residual, two patients (25%) had a significant re-hemorrhage requiring surgical evacuation; another 3 patients (37.5%) suffered from re-hemorrhage but did not require surgery; however, anticoagulation therapy was held again. In total, 62.5% in that group suffered from a re-hemorrhage. In the group with a small remnant, 2 patients (22.2%) had a re-hemorrhage, with one of the 2 (11.1%) requiring neurosurgical intervention. A large remnant was associated with an increased risk of re-hemorrhage (P = 0.04). For the entire group with a residual SDH, 41.2% (7 patients) suffered a SDH re-hemorrhage with 17.6% (3 patients) requiring surgical intervention. One patient remained with mild expressive dysphasia after a re-hemorrhage, despite surgical evacuation of the expanding SDH. The remaining dysphasia, however, was mild.
Discussion
The present study gives information about the risk of anticoagulation therapy for patients with traumatic SDH. Traumatic intracerebral contusion, subarachnoid hemorrhage, or epidural hematoma do not tend to progress after a few days post-injury and most expert will agree that initiating anticoagulation therapy after about one week post-injury is safe in regards to the TBI. SDH, however, presents a challenge to the clinician when patients are on anticoagulants, as SDH tends to re-hemorrhage long after the initial event. Reference Bajsarowicz, Prakash and Lamoureux9 Anticoagulants are commonly used, and their beneficial effects in preventing thromboembolic events are well documented, especially for patients with mechanical cardiac valves Reference Cannegieter, Rosendaal and Briët4 or atrial fibrillation. 3 However, these pathologies are more common in the elderly population – a population that is also at increased risk of falls and TBI. Gaining a better knowledge on the safest use of anticoagulant in traumatic SDH is therefore of prime importance.
Our study showed that a temporary interruption of anticoagulant therapy, for an average duration of 105.6 days and a median of 67 days, led to a 1.1% thromboembolic complication rate. The complication was reversible in that particular patient. It also showed that initiation of anticoagulant therapy, while the SDH is still not completely resolved, is associated with a risk of re-hemorrhage of 41.2% and a risk of requiring a surgery of 17.6%. This risk is 3.5 times higher than if there was no anticoagulant therapy when comparing the rate of re-hemorrhage in a cohort study from our institution (5%). Reference Bajsarowicz, Prakash and Lamoureux9 For one patient (5.9%), there was permanent but mild neurological deficit related to the re-hemorrhage. The risk of re-hemorrhage was significantly higher if the residual SDH was large compared to small.
Many studies have looked at anticoagulation in neurosurgical pathologies, but only a few have looked at TBI. Reference Pandya, Pattison, Karas and O’Mara10,Reference Byrnes, Irwin, Roach, James, Horst and Reicks16–Reference Wijdicks, Schievink, Brown and Mullany19 The American Heart Association and the American Stroke Association Stroke Council formulated guidelines for patients with spontaneous intracranial hemorrhage and stated that anticoagulation interruption for 7 to 10 days did not result in thromboembolic complications and was therefore safe. Reference Broderick, Connolly and Feldmann20 The guidelines do not refer to traumatic lesions, however, and while many TBI patients are in a hypocoagulable state, some patients are hypercoagulable in the acute setting. Furthermore, the risk of re-hemorrhage is not addressed. A recent study showed that the probability of having thromboembolic complications in patients with high risks factors (high CHADS2 score or mechanical heart valves) was non-existent for the first 7 days without anticoagulation. The risk however increased from 0% at 7 days to 10.5% at 14 days, and at 38.5% at 30 days. This study included only 6 SDH, and the anticoagulants were held for an average of 57 days. There is no mention of the status of the SDH at the time of anticoagulation resumption. Reference Jung, Jeon, Chang and Jung17 Pandya et al. included 55 patients with SDH and anticoagulation or antithrombotic therapy. They showed 9,1% risk of “clinically significant” hematoma expansion when anticoagulation or antithrombotic therapy was initiated within 14 days of admission. The size of the SDH at the time of anticoagulation initiation was not mentioned, and the total observation time was also not mentioned. Reference Pandya, Pattison, Karas and O’Mara10 Another study included 11 chronic SDH, where anticoagulation was held from less than 3 days to more than 1 month. None of the SDH re-hemorrhaged; however, there was, once again, no more information on the status of the SDH at the time of anticoagulation resumption. Reference Kawamata, Takeshita, Kubo, Izawa, Kagawa and Takakura18
Wijdicks et al. Reference Wijdicks, Schievink, Brown and Mullany19 looked at patients anticoagulated for mechanical heart valves and with intracranial hemorrhage. Thirty-nine patients were included, among which 20 had acute SDH, 10 had lobar hematomas, 4 had subarachnoid hemorrhage, 3 had cerebellar hematomas, and 2 had basal ganglionic hematomas. Thirteen patients died within 2 days from their intracerebral hemorrhage while the remaining 26 had no thromboembolic events for the duration of anticoagulation therapy interruption, which lasted on average 8 days and ranged from 2 days to 3 months. Wijdicks et al. Reference Wijdicks, Schievink, Brown and Mullany19 therefore suggested that a temporary interruption of anticoagulation, including aggressive reversal upon presentation, in those patients with intracranial hemorrhage and mechanical heart valves with no history of systemic embolization was safe. Their recommendation was that a discontinuation of anticoagulation for 1 to 2 weeks should be considered to follow the evolution of the hematoma. No patient developed expansion of their intracranial hemorrhage once anticoagulation had been restarted, but SDH patients were not separately analyzed and there was no mention on the amount of residual hematoma either.
Another study looked at TBI patients specifically and post-traumatic DVT and PE. Byrnes et al. Reference Byrnes, Irwin, Roach, James, Horst and Reicks16 found 42 patients for which anticoagulation was indicated to treat post-traumatic thromboembolic events. Twenty-six were anticoagulated, while 16 were not. The average timing to start anticoagulation was 12 days post-trauma, with 23 % receiving anticoagulation within 1 week of the trauma, and 31% after 2 weeks only. Half of the 26 patients (13) had an initial diagnosis of SDH. None of the SDH expanded in that study; however, the size of the SDH at the time of initiation of anticoagulation is again not mentioned.
All the studies presented had a relatively small number of patients with traumatic SDH (6, 11, 20 and 13). Reference Byrnes, Irwin, Roach, James, Horst and Reicks16–Reference Wijdicks, Schievink, Brown and Mullany19 Our study has the largest sample in the literature so far on the one type of intracranial traumatic pathology (SDH) that causes the main dilemma. Our study showed a high risk of SDH re-hemorrhage with anticoagulation, while none of the previously mentioned studies had SDH re-hemorrhage. The timing of anticoagulation resumption varied in those studies from a few days to many weeks. The average delay for SDH in Jung et al. Reference Jung, Jeon, Chang and Jung17 was 57 days. However, none of the mentioned studies described the abnormalities on brain CT at the time of anticoagulation therapy resumption. There was also no description of how the follow-up and the detection of re-hemorrhage was done. Our study is the first to clearly show that the amount of time elapsed since stopping anticoagulation therapy is not as important as whether the SDH has resolved or not.
The guidelines from the American Heart Association and the American Stroke Association Stroke Council stipulated that interrupting anticoagulation for 7 to 10 days was safe for patients with indications for anticoagulation. Reference Broderick, Connolly and Feldmann20 Another study on prosthetic heart valve found that 2 weeks of anticoagulation interruption did not result in adverse events, Reference Ananthasubramaniam, Beattie, Rosman, Jayam and Borzal21 while another study including patients at high risk found that the probabilities of having thromboembolic complications increased with time (0.0% at 7 days, 10.53% at 14 days, and 38.49 at 30 days). Reference Jung, Jeon, Chang and Jung17 Our study had only 1.1% occurrence of thromboembolic complication, despite a prolonged interruption in anticoagulation for many patients. This could be explained by the fact that many of our patients did not have a strong indication for anticoagulation, or were not at high risk. Similar rates of thromboembolic events were reported in studies on chronic subdural and patients on antithrombotic agents prior to presentation and surgery. Reference Guha, Coyne and Macdonald22–Reference Zhang, Aw and Tan23
Limitations of the study
The main limitation of this study lies in the fact that it is a retrospective study. Some cases could have been missed simply because documentation and history taking was not thorough. Likewise, the CHADS2 scores were not well documented in the patients’ cohort and therefore could not be used to judge the exact risk of thromboembolic events in patients anticoagulated for atrial fibrillation. Also, some patients were lost to follow-up and could not be included; although since the cohort was mainly composed of elderly patients and patients with chronic medical diseases who have regular medical follow-up, the number of patients who did not come for follow-up remained relatively low (4 patients-4.2% of the cohort). Therefore, an inclusion bias towards patient who had complications might have occurred. Likewise, the possibility of an acute and severe deterioration leading to death in patients lost to follow-up cannot be excluded, particularly if patients presented to an outside facility and were never transferred to our institution. Finally, the number of patients with residual SDH who were treated with anticoagulants is very small, and therefore, results can easily be skewed.
Recommendations
For the majority of the patients, the safest course is to wait until the SDH has resolved before initiating anticoagulant therapy. However, the SDH should be closely followed so that the medication can be started as soon as possible. A CT scan can be done as early as 1-2 weeks post-injury for small SDH. Larger SDH may take somewhat longer to resolve but the majority will have resolved by 2-3 months, since half of the cohort had a normalized CT by 67 days. Some patients required a much longer time for their SDH to resolve but for some others in our cohort, the follow-up could have been done earlier.
Patients at higher risk of thromboembolic events, such as those with higher CHADS2 score, or metallic mitral valve replacement will be harder to manage. A re-hemorrhage in the SDH may have less dramatic consequences than a large territory stroke. It might therefore be wise to restart anticoagulation earlier, combined with a close follow-up of the SDH. These patients could also benefit from follow-up cardiac ultrasound to detect the presence of an atrial clot or a thrombosed valve. This is what was done for the patient in our cohort who was found to have an atrial clot. Fortunately, it was diagnosed before sending emboli and causing a potentially devastating stroke. However, one must not forget that if there is a SDH re-hemorrhage, anticoagulation therapy will often have to be held again and perhaps for a longer period. An aggressive approach to the SDH with surgical evacuation to reduce the time delay for its resolution might be justified in some patients, provided that their surgical risk is not too high. This approach was also suggested by Kawamata et al. Reference Kawamata, Takeshita, Kubo, Izawa, Kagawa and Takakura18
Conclusion
This study on the risk of anticoagulation therapy in the setting of traumatic SDH shows that while a SDH is still present, the risk of re-hemorrhage is 41.2%, and the risk of requiring a surgery for the re-hemorrhage is 17.6%. The risk of re-hemorrhage increases when the residual SDH is larger. Withholding anticoagulant therapy for a median of 67 days was associated with a 1.1% incidence of adverse thromboembolic events. Regular imaging to follow the SDH regression, as well as close monitoring of patients with higher risk of thromboembolic events is warranted.
Acknowledgments
The authors would like to thank Dr Abdullah Al-Kuweiti for his initiative in the project, Mrs. Mitra Feyz and Mrs. Johanne Prudhomme for their help with the database, the archivists for their time and effort, and Dr Mohammad Maleki for caring for these patients.
Disclosure of Interest
Rajeet Singh Saluja has received stat up funds from the Research Institute of McGill University Health and the Neurosurgery Department Practice Plan; none of those funds were used for the preparation of the present manuscript. He has also received payment for medico-legal expertise from The Canadian Medical Protection Association and from the Boro Gordon Frigon and Jones Law Firm, but none of those payments have any link to the present manuscript. The other authors have no conflict of interest to report.
Statement of Authorship
Maryam Kia collected and analyzed data, drafted the manuscript, and critically reviewed it.
Judith Marcoux supervised the project, collected data, and completed the manuscript.
Rajeet Singh Saluja critically reviewed the manuscript.





