INTRODUCTION
Antidotes can be an essential part of the emergency management of poisoned patients. Inadequate antidote stocking in hospitals is a well-documented occurrence on an international level. Several survey studies in different countries have documented inadequate stores of and accessibility to time-sensitive antidotes.Reference Juurlink, McGuigan, Paton and Redelmeier1-Reference Mitchell, Higginson and Smith14 Canadian studies have also highlighted the scope of this problem in British Columbia (BC), Ontario, and Quebec.Reference Juurlink, McGuigan, Paton and Redelmeier1-Reference Bailey, Bussières and Dumont6 The BC group published consensus guidelines for antidote stocking as a response to this issue and provided strategies for appropriate population- and hospital-based distribution. A follow-up survey demonstrated improved yet suboptimal compliance with recommendations.Reference Wiens, Zed and Lepik5, Reference Bailey, Bussières and Dumont6
Potential reasons for these deficiencies included cost, a lack of awareness of the importance of antidotes, the absence of national standards, hospital size, and antidote availability.Reference Gorman, Zed, Purssell, Brubacher and Willis2-Reference Sivilotti, Eisen, Lee and Peterson4 Certain antidotes are not readily available in Canada and require procurement through the Special Access Program (Health Canada) that may present a challenge for some hospitals. These barriers are more significant for smaller hospitals that have limited resources.Reference Juurlink, McGuigan, Paton and Redelmeier1-Reference Bailey and Bussières3
A solution to inadequate antidote stocking and accessibility was presented in one publication in which a toxicology cart with all necessary antidotes within an emergency department (ED) was implemented.Reference Pettit, McKinney, Achusim and Lindsey16 The Nova Scotia (NS) program has a similar premise but was extrapolated to an entire province. A 2004 local antidote stocking surveyReference Dart, Borron and Caravati7 based on consensus guidelines was conducted in EDs in the district that contain both adult and pediatric tertiary care centres. It revealed deficiencies similar to those in the literature, indicating an overall compliance of 62.5% for 14 of the currently recommended antidotes (Appendix A). This provided the impetus for a comprehensive district-wide pilot program, which resulted in 100% compliance with the antidote stocking guidelines at all eight sites. The pilot program then evolved into a province-wide program in 2009. Guidelines for antidote stocking were developed for the province, but the main features of the program that differed from other approaches in the literature are as follows:
- Placement of a highly visible room temperature antidote kit (Figure 1) and refrigerated antidote kit within each ED.
- Surveillance and maintenance of the kits in each district by a designated pharmacy.
- Antidote-specific administration instructions available online.
- Support from provincial hospital administration.
- Antidote-specific data collection obtained through real-time surveillance through poison centre consultations.
- Biannual interdisciplinary antidote committee meetings.
- Ongoing communication with ED chiefs, pharmacy directors, and ED nurse educators.

Figure 1. (a) The antidote kit. (b) Three tiers of standardized antidotes.
This paper outlines the complex process of planning and implementing such a program and describes antidote utilization patterns over an 11-year period in NS.
METHODS
This section outlines the methodology for antidote program development and implementation, followed by the methodology for data extraction and analysis. This study was approved by the research ethics board at the IWK Health Centre, in Halifax, NS.
Methods part one: Antidote program development and implementation
A multidisciplinary committee including a medical toxicologist, an emergency physician, pharmacists, pharmacy technicians, and a poison centre specialist was established. Five objectives were identified and are described below.
1. Approval and funding
Approval was obtained from the provincial hospital administration, and hospitals were encouraged to commit to the program; however, no dedicated provincial funding for this program was secured. Costs were absorbed by the pharmacy and EDs in each district. The costs included procurement and maintenance of antidotes and a room temperature and refrigerated storage box to house antidotes in EDs, as well as sharing the salary cost of a provincial antidote coordinator. The procurement costReference Sivilotti, Eisen, Lee and Peterson4 for recommended antidotes in 2005 was approximately $9,600 and is currently estimated to be $11,760.
2. Antidote stocking guidelines
The committee used the current literature and advice from expert consultants to determine recommended antidotes. Recommended quantities were based on the estimated 24-hour treatment supply for a 70-kg patient, accessibility to other sites for rarely required or expensive antidotes, the likelihood of use, expert opinion, and cost. Geographic distribution of provincial EDs allowed sharing of antidotes. Antidotes that were designated ward stock antidotes were generally stocked in larger quantities as they could be used for indications other than toxicity.
Two types of antidote kits were developed: a “full” kit containing original antidote quantities and a “modified” kit for smaller EDs with quantities that provided four hours of treatment. Current recommendations for antidotes and quantities in NS are listed in Table 1.
Table 1. Antidote stocking recommendations in NS
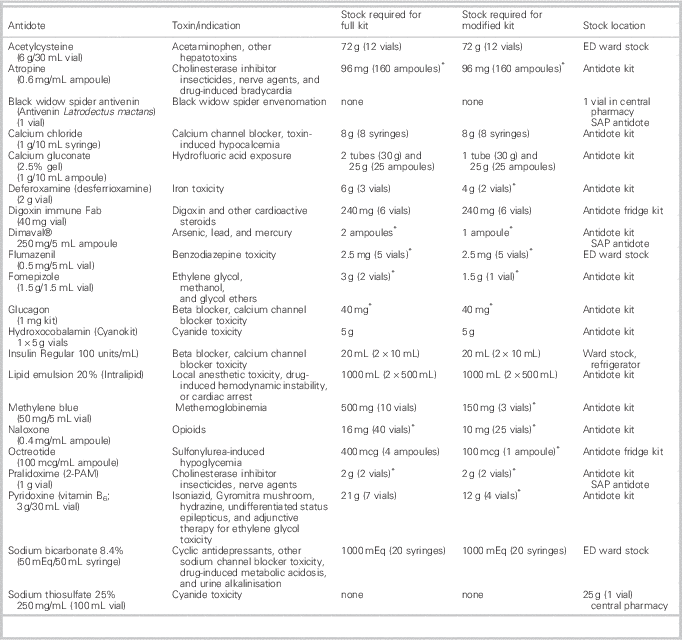
* In the event of an overdose, additional stock will be required to treat a patient for 24 hours
The designated central pharmacy for the program was located at the adult tertiary care centre. This pharmacy stocks two rarely used antidotes, sodium thiosulfate and black widow spider antivenin. The program recommends three antidotes available through the Special Access Program (SAP; black widow spider antivenin, Dimaval®, and pralidoxime). The central pharmacy is benefitted by an SAP pharmacy technician who procures SAP antidotes for that district and assists other districts as required.
3. Access to antidotes
A three-tiered yellow plastic box containing most of the antidotes (Figure 1) enhanced the visibility and accessibility of antidotes within EDs. Four antidotes were designated as ED ward stock and not included in the kits (Table 1). Antidotes requiring refrigeration were placed in a small labelled red box and located in a designated ED refrigerator.
4. Antidote administration guidelines
Evidence-informed antidote-specific monographs were developed in a standard format with information including toxicologic indications, dosing specific to antidote use, detailed preparation and administration guidelines, adverse effects, intravenous (IV) compatibility, and stability. These monographs were developed and maintained by the poison centre and are located on their website.
5. Maintenance and surveillance of the program
Stocking and replenishment
For the purposes of the program, NS was divided into nine geographical districts. Stocking and replenishment were the responsibility of the pharmacy director at each district site and was typically carried out by a trained pharmacy technician. Although specific pharmacy procedures may differ, kits are assembled at one designated district hospital and dispensed to EDs as a sealed entity with an expiry date. Each district has a policy and procedure for restocking kits in instances in which an additional supply of an antidote or antidotes are required immediately, after a kit is used, and when the stock is required on an urgent basis.
To facilitate cost containment, central tracking of expiry dates is performed in each district, and antidotes are recycled prior to expiry if possible (e.g., naloxone to operating rooms). If recycling is not successful, expired products are returned to the manufacturers for credit as permitted. For antidotes designated as ED ward stock (Table 1), a quality assurance survey is conducted annually.
Education
Education was provided by the provincial antidote coordinator to ED staff across the province. The program was publicized through multiple continuing medical education (CME) activities in the region and in written communications with ED chiefs, pharmacy directors, and ED nurse educators.
Management and data collection
The provincial antidote committee was responsible for management of the program and met biannually. The committee reviewed utilization data, advised on issues of drug procurement and shortages, and incorporated new evidence into recommendations. The provincial antidote coordinator disseminated quarterly communications about the program to provincial stakeholders.
Antidote surveillance was recorded in a database with data collected from calls to the poison centre and direct communications with hospital pharmacists. Information collected included patient identifiers, site, date, indication, antidote(s) used, and adverse events, as well as an assessment of the final medical outcome (no effect, minor effect, moderate effect, major effect, or death, as per the American Association of Poison Control Centers [AAPCC]).Reference Mowry, Spyker, Brooks, McMillan and Schauben15
Methods part two: quantitative analysis of compliance with stocking recommendations and antidote usage
Study design
This is a retrospective, non-comparative, and descriptive study.
Data sources and collection
The IWK Regional Poison Centre in Halifax, NS, serves a population of approximately 1.2 million people and receives approximately 9,000 calls annually. Health care in NS is delivered by 2 tertiary care hospitals, 9 regional hospitals, and 25 community hospitals providing emergency care. The tertiary care hospitals have 912 (adult) and 90 (pediatric) acute care beds; regional hospitals have a median of 103 acute care beds (range 73-436); and all community hospitals have less than 50 acute care beds.
Antidote utilization data for this study were extracted from the database maintained by the provincial antidote coordinator. Compliance with the antidote program was determined by direct communications with pharmacy directors at least annually in each district from May 2005 to May 2016.
The following three fields were extracted from the database for each case in which an antidote was administered: antidote used, the size of the hospital (tertiary, regional, or community), and the medical outcome as per the AAPCC.Reference Mowry, Spyker, Brooks, McMillan and Schauben15
Outcome measures
1. Hospital compliance with antidote stocking recommendations.
2. Antidote utilization from May 2005 to May 2016, including type and frequency of antidote use and distribution of antidote use across hospital types.
3. Patient outcomes, as defined by the AAPCC.
Data analysis
Descriptive statistics were used to summarize the data using Microsoft® Excel.
RESULTS
Compliance with the program
From 2005 to 2009, the pilot project in the largest health district, which included the only adult and pediatric tertiary care EDs, a regional ED, and five community EDs, showed 100% compliance. After provincial expansion in 2009, 7 of the 9 regional EDs and 8 of the 25 community EDs were compliant. By 2011, all nine (100%) regional EDs were compliant; by 2013, 21 of the 25 (84%) community EDs were compliant (Appendix B).
The overall compliance with the program is 89% (32/36) of the provincial EDs that was achieved during a four year period. No EDs have withdrawn from the program.
Antidote utilization and distribution
A complete description of usage patterns is presented in Table 2. It should be noted that use of N-acetylcysteine (NAC) was not officially tracked by the program because it has been consistently well stocked. To establish context, there were 1,257 reported cases in which NAC was used during the study period.
Table 2. Antidote utilization, 2005-2016
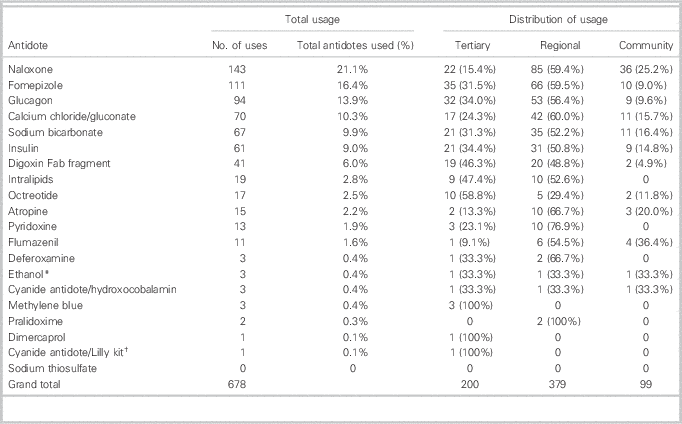
*Antidote previously recommended by the NS antidote program
†Lilly kit was initially recommended; hydroxocobalamin is the current recommendation
From May 2005 to May 2016, a total of 678 antidotes were used for 520 patients. Of the 19 recommended antidotes (excluding NAC and grouping calcium formats), 17 were used during the study period. The five most commonly used antidotes were: naloxone (143/678, 21.1%), fomepizole (111/678, 16.4%), glucagon (94/678, 13.9%), calcium chloride or gluconate (70/678, 10.3%), and sodium bicarbonate (67/678, 9.9%). Antidotes considered to be rarely indicated were also used, including dimercaprol, pralidoxime, and hydroxocobalamin. Antidote utilization by hospital type was as follows: 99/678 (14.6%) at community hospitals, 379/678 (55.9%) at regional hospitals, and 200/678 (29.5%) at tertiary care hospitals (Table 3).
Table 3. Antidote use by hospital type

Patient outcomes
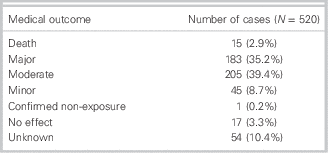
Outcome definitions were based on standard criteria established by the AAPCC.Reference Mowry, Spyker, Brooks, McMillan and Schauben15 Of the 520 patients for whom antidotes were used, death occurred in 15 of the 520 (2.9%), major outcomes (e.g., life-threatening effects such as hemodynamic instability) occurred in 183 of the 520 (35.2%), moderate outcomes (e.g., not life-threatening but requiring treatment, such as isolated seizure) occurred in 205 in the 520 (39.4%), minor outcomes (e.g., transient, mild symptoms) occurred in 45 in the 520 (8.7%), and no effect occurred in 17 in the 520 (3.3%). The outcome was unknown in 54 of the 520 patients (10.4%), and one case was confirmed as a non-exposure case (Table 4).
Table 4. Medical outcomes of antidote cases
DISCUSSION
The NS antidote program represents a tangible solution to the problem of inadequate antidote stocking and is, to our knowledge, the first of its kind. This program demonstrates that concrete and sustainable antidote stocking is achievable but requires a system-wide approach to ongoing maintenance and surveillance by a multidisciplinary group. Despite the lack of dedicated funding, overall compliance of 89% was achieved during a four year period and has been sustained since 2013, and no ED has dropped out of the program once involved.
The goal of this program is to ensure that all EDs have immediate access to time-sensitive antidotes, regardless of hospital size and geographic location. The cost for each individual hospital to be compliant with currently published stocking guidelines may represent a significant financial burden. Our program mitigates the cost of maintaining antidote stocks while considering patient safety by establishing a network of antidotes over large geographic areas. Mechanisms for the sharing of antidotes are crucial to this model and should be developed by hospital pharmacies and EDs in the event that larger doses of antidotes are required for a specific case.
The procurement cost of antidotes is a barrier to stocking, but this must be weighed against other, possibly more significant costs arising from poisonings that are not rapidly reversed with an antidote. Such costs may include those associated with the treatment of toxin-induced renal failure or another organ injury, prolonged hospital stays, patient transport, or extracorporeal methods of toxin removal. In our experience, cost has been a concern for hospital pharmacies and EDs. Efforts to contain costs were made by recommending a “modified kit” (Table 1) for smaller EDs, recycling antidotes to other areas of the hospital prior to expiry, and returning antidotes to manufacturers for credit if possible. In addition, antidote utilization patterns have informed alterations to antidote stocking recommendations. For example, pralidoxime was stocked in all antidote kits; based on our data showing rare usage, as well as the fact that atropine is the initial treatment in cholinergic toxidrome, we will be recommending that it be stocked only at regional hospitals while maintaining an overall provincial stock that could still treat a patient for a few days. Additional cost reductions have been achieved by reducing the glucagon stock. Glucagon is expensive: if the maximum dose of 10 mg/hour is administered, it costs approximately $8,000 per 24 hours. New evidence-based consensus guidelines do not include glucagon as an antidote for calcium channel blocker (CCB) toxicity,Reference St-Onge, Anseeuw and Cantrell21 and it is typically used as only adjunctive therapy, if at all, in beta-blocker (BB) toxicity.Reference Graudins, Lee and Druda22 Insulin is supported in the literature as an antidote for both CCB and BB toxicity and is inexpensive (approximately $50 per 24 hours). Maintaining an appropriate supply of insulin while reducing glucagon is a cost-effective approach that is supported by the evidence available. Digoxin immune Fab fragments were rarely used by community EDs during the study period. This antidote is still required for immediate treatment of digoxin toxicity, but the stocking recommendations for community hospitals will be reduced in our program as a result. This is supported by recent studies, which have called into question the current empiric dosing of digoxin immune Fab fragments as being overestimated and have documented that calculated doses are low.Reference Chhabra, Valento, Bryant and Aks17-Reference Chan and Buckley20 This antidote costs approximately $850 per vial, which has doubled since 2005 and is no longer returnable if unused. The current stocking recommendations of six vials per site for our program already reduces this cost to some extent by providing each ED the ability to initiate treatment. Further stock can be obtained as needed. In summary, by monitoring the utilization data and regularly evaluating the evidence, costs can be reduced while preserving patient safety.
The program did increase workload within hospital pharmacies, and compliance with the antidote stocking guidelines placed a financial burden on ED and pharmacy budgets for drug procurement. At some point, this may not be sustainable without dedicated funding, and we recommend that this issue be addressed during the planning stages of any similar program.
A small hospital size has been described as a barrier to sufficient antidote stocking because of limited resources, a lack of specialist physicians, and the perception that treating poisoned patients is rare. In NS, 70.5% of antidote usage during the study period was by regional and community EDs. This contradicts the assumption that smaller hospitals do not need to stock “expensive” antidotes. The frequency of antidote use was higher than expected, every six days (excluding NAC), on average, which supports the necessity of stocking antidotes for emergency preparedness.
Most patients who required antidotes had a final recorded outcome demonstrating important morbidity and mortality information. These patients presented to the community and regional EDs in significant numbers. Our data support that having ready access to time-sensitive antidotes in these hospitals is an essential aspect of hospital emergency preparedness.
The need for consistent preparation, administration, and dosing guidelines was evident at the inception of the antidote program. Most hospital IV manuals contained information about only the non-antidotal use of medications such as insulin or glucagon. In addition, there is a multitude of resources with varying recommendations, with no step-by-step preparation instructions. In our opinion, dedicated antidote administration guidelines are essential while implementing an antidote program for consistency, clarity, and timely administration.
Poison centre involvement proved essential to the NS antidote program. Poison centre staff promote awareness of the antidote program and administration guidelines while managing cases in real time with on-call physician backup. Poison centres are the only health care service to provide continuity of care for the poisoned patient from the time a call comes from the home to prehospital, ED, and intensive care; thus, poison centres are vital for optimizing care for this patient population.
NS was an ideal province for such a pilot program, given its relatively small geographic area and population densities, as well as its infrastructure including a poison centre, an advanced prehospital system, and two tertiary care centres serving adult and pediatric populations. The basic principles of this program could be extrapolated to larger provinces or countries to achieve success in antidote stocking.
LIMITATIONS
Antidote and patient tracking in our program relied on voluntary reporting through the poison centre, hospital pharmacies, and/or hospital pharmacy inventory systems. In addition, NAC use was not included, and it was more difficult to track antidotes not contained in the kit. Antidotes with which clinicians have a high level of comfort (e.g., naloxone) may not prompt a call to the poison centre. Therefore, actual antidote usage is undoubtedly much larger than what was reported. This study was not designed to evaluate whether antidote use was appropriate, any potential relationship between antidote stocking and patient outcome, or cost-effectiveness. Future research will examine these issues.
Another limitation was that compliance with the antidote program is not mandatory. Each district used its discretion regarding antidote kit stocking; as a result, implementation took years to achieve. Nonetheless, overall compliance is currently excellent.
CONCLUSIONS
Front-line healthcare providers need timely access to necessary antidotes, regardless of geographic location, and standardized, up-to-date guidelines for antidote administration. The NS antidote program is, to our knowledge, the first large scale program to demonstrate that a concrete and sustainable solution to ED antidote stocking is achievable with a system-wide approach and ongoing maintenance and surveillance. The frequency and distribution of antidote use in this program support the need for adequate antidote stocking in all EDs regardless of hospital size. Poison centres provide consultations to large geographic areas through telecommunication and are uniquely poised to support surveillance and improvement of an antidote program.
Acknowledgements
The authors would like to acknowledge the following people who have made contributions to this project: Mark Fletcher, Michael Howlett, Gisia Pisegna, L. Nicky Corkum, Catherine Doherty, Ardeth Reardon, Vanessa Sherwood, Laurel Ross, Patty Timms-Cluett, Peter MacMillan, Kathy Hart, David Juurlink, Wenda MacDonald, Peter Zed, and Jill Duncan.
Competing interests
None declared.
APPENDIX A. LOCAL ANTIDOTE STOCKING SURVEY BEFORE IMPLEMENTATION OF THE ANTIDOTE PROGRAM (2004)

APPENDIX B. COMPLIANCE OF EDS WITH THE NS ANTIDOTE PROGRAM BY YEAR, HOSPITAL TYPE, AND ANTIDOTE KIT TYPE








