INTRODUCTION
Atrial fibrillation and flutter (AFF) are the most common arrhythmias presenting to the emergency department (ED) and are associated with significant morbidity and mortality.Reference Feinberg, Blackshear and Laupacis 1 - Reference Wolf, Abbott and Kannel 3 Chronic atrial fibrillation increases stroke risk,Reference Lakshminarayan, Anderson and Herzog 4 - Reference Albers, Atwood and Hirsh 6 and individuals with paroxysmal atrial fibrillation may have a similar prognosis.Reference Hohnloser, Pajitnev and Pogue 7 , Reference Nieuwlaat, Dinh and Olsson 8 In the United States, over 60% of ED patients with primary AFF are admitted to the hospital,Reference McDonald, Pelletier and Ellinor 9 whereas 15% of Canadian patients are admitted.Reference Scheuermeyer, Grafstein and Stenstrom 10 Given that many patients may have difficulty accessing their family physicians or consultants, emergency physicians (EPs) may play a crucial role in providing evidence-based care to optimize patient outcomes.Reference Scheuermeyer, Grafstein and Stenstrom 10 - Reference Michael, Stiell and Agarwal 13
Embolic stroke is a complication of AFF, and studies have shown that many ED patients arrive with inappropriate anticoagulation.Reference Misra, Lang and Clement 14 , Reference Scheuermeyer, Innes and Pourvali 15 Unfortunately, EPs miss many opportunities to initiate anticoagulation in at-risk patients at the time of their ED visit.Reference Misra, Lang and Clement 14 , Reference Scheuermeyer, Innes and Pourvali 15 This misapplication of clinical practice guidelines is troubling, and the reasons are unclear.
To improve patient care, a coordinated, evidence-based, ED-based AFF pathway was developed at our institution. The primary objective of this study was to measure rates of new appropriate anticoagulation on ED discharge for AFF patients who were previously not anticoagulated according to evidence-based guidelines. Secondary objectives included evaluation of additional patient- and system-specific outcomes, including length of stay (LOS), rates of congestive heart failure (CHF), major bleeding complications and stroke at 30 days, referrals to our institution’s AFF clinic, and ED revisit rates.
METHODS
This pre-post program evaluation chart review was conducted at two university-affiliated urban sites. St. Paul’s Hospital is a tertiary care, inner-city hospital with an annual census of 84,000 ED visits. Cardiac care is comprehensive and includes a 24-hour catheterization laboratory, an electrophysiology service, and cardiac transplant capability. Mount St. Joseph’s hospital is a community centre receiving 35,000 ED patients annually and a general internal medicine ward. The centres share an administrative database capturing patient arrival and discharge times and all orders, including investigations, results, treatments, and consultations since 1999. This is linked to a validated regional database to capture ED revisits and to provincial vital statistics to capture mortality.Reference Scheuermeyer, Grafstein and Stenstrom 10 This study was approved by the Clinical Research Ethics Board for the University of British Columbia.
Intervention
A coordinated, evidence-based ED atrial fibrillation and atrial flutter pathway was developed by EPs (CD, EG) in collaboration with cardiologists (BH, ST) and pharmacists (JH) at our institution. Pathway content was finalized through consensus discussion between emergency medicine, cardiology, and pharmacy (CD, BH, ST, JH). The pathway consists of a care map, decision aids, medication orders, management suggestions, and electronic consultation or referral documents, all embedded into the computerized physician order entry and integrated electronic medical record program (Sunrise Clinical Manager version 4.0, Eclipsys Corporation, Atlanta, Georgia). This program includes access to clinical decision support scores (e.g., CHADS2 and HASBLED) and decision aids (Appendix 1). Implementation was preceded by a 1-month period, including educational rounds and targeted emails. Emails included background, rationale, and education on key elements of the pathway and were sent to EPs during 1 month, and 1 week prior to pathway implementation, and weekly for 1 month following implementation.
Patient selection
Using the database, we identified consecutive patients presenting to the two study EDs with a final EP diagnosis of atrial fibrillation (ICD10 code I48.0) or flutter (ICD10 code I48.1). Board-certified cardiologists verified each electrocardiogram (ECG) within 24 hours, and each patient required ECG confirmation of the diagnosis. The AFF pathway was activated at our institution on July 24, 2013. We included patients from April 30 until July 23, 2013 (pre-pathway implementation period [PRE]) and patients in the post-pathway group (POST) from August 25 until December 22, 2013. We allowed a 1-month education and implementation period for EPs to adopt the pathway (July 24 to August 24, 2013).
Exclusions
We enrolled uncomplicated AFF patients solely managed by EPs. The following patients were excluded: 1) those referred directly to cardiologists or internists in the ED because EPs would not manage anticoagulation; 2) those attending the ED only to monitor their anticoagulation; 3) those within 7 days of invasive cardiac procedures (pacemaker implantation, ablation procedures, coronary artery bypass grafting, or percutaneous coronary intervention) as management decisions are deferred to the surgeon or cardiologist; and 4) those with the following acute medical conditions: sepsis, shock, pneumonia, acute coronary syndrome, acute decompensated CHF, pulmonary embolism, chronic obstructive pulmonary disease (COPD), thyrotoxicosis, hypertensive emergency, drug overdose, acute valvular disease, or hypothermia. (See Appendix 2 for standardized definitions.)
Chart review
We adhered to the criteria for medical record review described by Kaji et al.Reference Kaji, Schriger and Green 16 Six senior medical student reviewers, blinded to study hypothesis and patient outcomes, and trained on the first 10 charts, independently abstracted charts onto standardized electronic spreadsheets to document triage scores, comorbidities, and ED treatments. Patients’ electronic charts were scrutinized as far back as 1999 to clarify missing or unclear information; such controversial data were managed by external audit by the primary investigator (DB). A random sample of 20% of the data was double-collected by reviewers blinded to the initial data and checked by study investigators to ensure quality and completeness. Inter-rater reliability was assessed on a random subset of 75 charts (24.9%) using the following variables: time to first ECG, cardioversion method, anticoagulation at ED arrival, and anticoagulation at ED discharge.
Data
AFF quality of care measures was determined from the Canadian Cardiovascular Society Guidelines for the management of AFF and consensus statements on quality of care in Canadian EDsReference Schull, Guttmann and Leaver 17 - Reference Skanes, Healey and Cairns 19 : rates of anticoagulation; other medication usage; ED cardioversion; ED LOS; and referral to outpatient atrial fibrillation were collected. (The hospitals share a referral clinic where patients are seen within 7-10 days by an electrophysiologist.) “New appropriate anticoagulation” was defined in this study as patients with atrial fibrillation or atrial flutter presenting to the ED who were not previously on antiplatelet or anticoagulant medications (refer to Appendix 1 for definitions of comorbidities and clinical scores [i.e., CHADS2]) and discharged from the ED with a prescription for new antiplatelet (aspirin [ASA] only)Reference Skanes, Healey and Cairns 19 or anticoagulant medications, depending on predefined prescribing thresholds for these medications (i.e., CHADS2) and risk of bleeding (HASBLED score), previously defined by the Canadian Cardiovascular Society.Reference Skanes, Healey and Cairns 19
Outcomes
The primary outcome is the proportion of AFF patients who were correctly started on new anticoagulant medications by the EP (based on CHADS2 and HASBLED) in each of the two time periods. Secondary outcomes for this study included ED LOS, outpatient clinic referral, and 30-day rates of the following: ED revisit, re-hospitalization, CHF, ischemic or hemorrhagic stroke, major bleeding, and death. Additional secondary outcomes included age (>65 years) and gender-based differences in the rates of new anticoagulation. Prior work on this topic has described age and gender discrepancies in the rates of new anticoagulation.Reference Béjot, Ben Salem and Osseby 5
Sample size calculation
Sample size was calculated a priori based upon the primary outcome. Upon reviewing the literature,Reference Scheuermeyer, Grafstein and Heilbron 11 , Reference Scheuermeyer, Innes and Pourvali 15 we anticipated our baseline rate of appropriate anticoagulation at discharge to be 34%. We determined a 15% change in the rates of new anticoagulation upon ED discharge as clinically significant through consensus discussion (RS, DH, DB), and an extensive review of prior work on this topic.Reference Scheuermeyer, Innes and Pourvali 15 , Reference Scheuermeyer, Mackay and Christenson 20 , Reference Buckingham and Hatala 21 To demonstrate a clinically important change of 15% in this proportion, with 80% power and an alpha of 0.05, we required 248 patients overall.
Statistical analysis
Proportions were compared using the chi-square test with 95% confidence intervals (CIs). Nonparametric data were compared using the Mann-Whitney U test with 95% CIs. Rates were described using proportions, medians, interquartile ranges (IQRs), and 95% CIs. We used Excel (version 14.4.8; Microsoft Corp., Redmond, Washington) and Stata (version 11; StataCorp, College Station, Texas) for all analyses.
RESULTS
The AFF pathway was activated at our institution on July 24, 2013. We included 129 patients from April 30 until July 23, 2013 (PRE) and 172 patients in POST from August 25 until December 22, 2013, for a total of 301 (Figure 1). The inter-rater agreement for data collection was k=0.81.
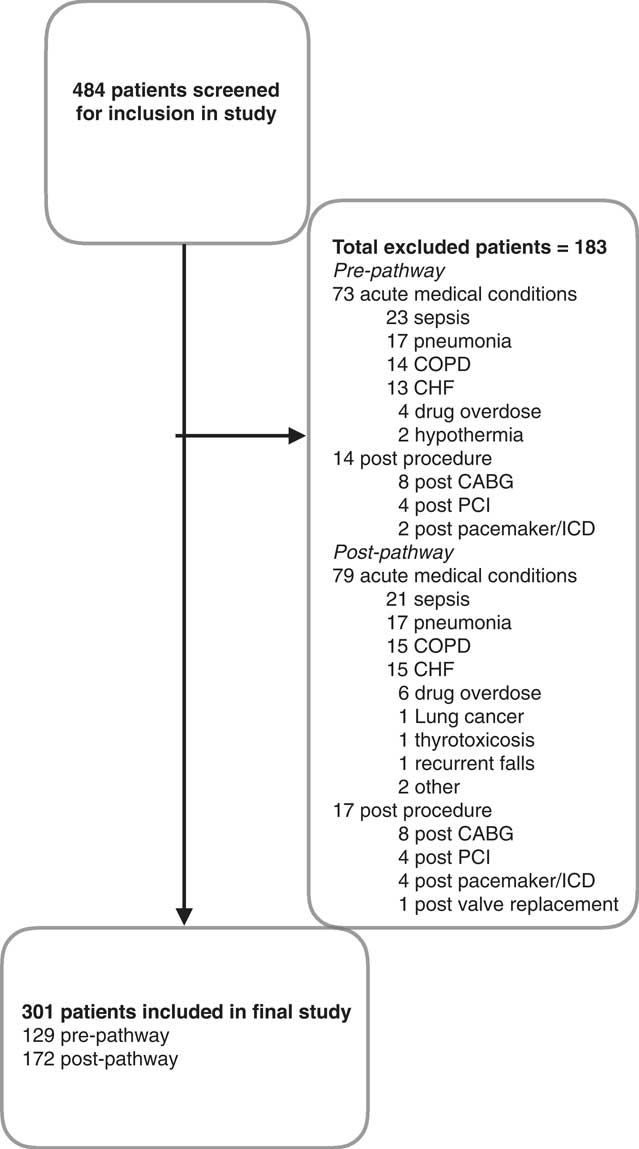
Figure 1 Patient flow within study
Baseline demographics of both groups were similar in terms of age, sex distribution, triage score, arrhythmia distribution, and comorbidities, evidenced by the similar CHADS2 and HASBLED scores. The rates of appropriate anticoagulation at ED presentation were 18.6% (PRE) and 19.7% (POST). Regarding ED management, there was a similar proportion of patients who were rate and rhythm controlled; consultation rates were likewise similar (Table 1).
Table 1 Descriptive characteristics of study population

CI=confidence interval; ECG=electrocardiogram; ED=emergency department.
* Canadian Triage and Acuity Scale.
† Atrial fibrillation or flutter with aberrancy, left bundle morphology or pacemaker.
The rates of new anticoagulation on discharge from the ED, for patients who were incorrectly not on anticoagulation at ED arrival, were 51 / 105 (48.6 %, 95% CI 42.1% to 55.1%) in the PRE group and 97 / 138 (70.2%, 95% CI 62.1% to 78.3%) in the POST group, for an absolute difference of 20.6% (95% CI 15.1% to 26.3%). The median age of patients receiving new anticoagulation was 61 years (PRE) and 65 years (POST), which were not significantly different.
Post-hoc analysis revealed that, in the pre-group, 10 / 53 women (18.6%) were on anticoagulation, comparable to the male rate. Of the 43 eligible women, none were started on anticoagulation, as compared to 69.7% in the male group. In the post-group, 22 / 56 eligible women (39.2%) (difference 39.2% [95% CI 25.1%-53.3%]) were started on anticoagulation, as compared to 75 / 102 eligible males.
The overall ED LOS for the departments did not change during the study periods (personal communication, D. Kalla). Median (IQR) ED LOS for patients with AFF was 262 minutes (162 to 431) in the pre-group and 218 minutes (152 to 375) in the post-group, for a difference of 44.0 minutes (p<0.03; 95% CI 36.2 minutes to 51.8 minutes) (Table 2). The rate of atrial fibrillation clinic referrals increased from 22 / 129 (17.1%) to 45 / 172 (26.2%) (difference 9.1%, 95% CI 5.85% to 12.35%). The new anticoagulation medications prescribed to patients by EPs in the PRE and POST study groups were similar (Table 3).
Table 2 ED AFF performance measures and outcomes
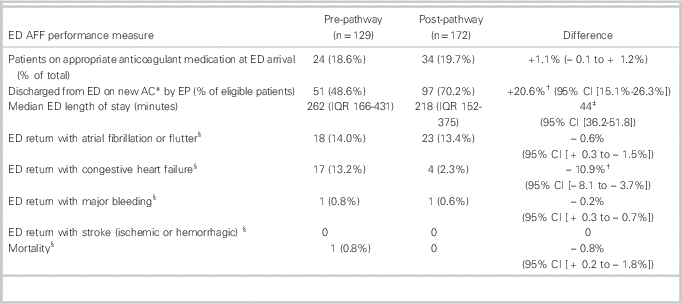
AFF=atrial fibrillation and flutter; CI=confidence interval; ED=emergency department; IQR=interquartile range.
* Appropriate anticoagulant or antithrombotic medication.
† p<0.01.
‡ p<0.03.
§ Within 30 days of index ED visit.
Table 3 Anticoagulant and antiplatelet medications used by study population
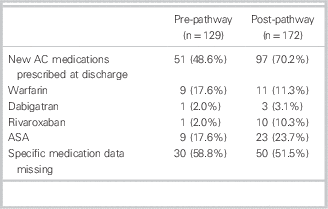
AC=anticoagulation; ASA=aspirin.
The 30-day ED revisit rate for CHF decreased from 13.2% (PRE) to 2.3% (POST) (absolute difference of 10.9%; p<0.01 (95% CI -8.1% to -13.7%). Thirty-day ED revisits for AFF, major bleeding episodes, and mortality were similar for the two groups. There were no strokes in either group. One study patient died: an 84-year-old male in the pre-group, with Stage IV liver cancer and long-standing atrial fibrillation. After discussion with his family, he was discharged home in stable condition and died at 28 days.
DISCUSSION
In this two-centre study of 301 patients with uncomplicated atrial fibrillation or flutter who were solely managed by EPs, the introduction of a standardized, evidence-based pathway dramatically improved rates of appropriate anticoagulation from 48.6% to 70.2%. In addition, without changes in the overall ED LOS at the two participating centres during the study period, the median ED LOS for patients with AFF decreased by 44 minutes. Furthermore, the 30-day ED revisit rate for CHF decreased from 13.2% to 2.3% following the implementation of our pathway. Importantly, rates of ED revisits for AFF, major bleeding, and mortality remained similarly low, there were no strokes in either group, and clinic follow-up rates improved. This assists clinicians by highlighting the benefits of a coordinated, evidence-based ED AFF pathway on improving care and potentially decreasing resource use.
Although new anticoagulation is a key quality of care indicator for patients with AFF by expert consensus statements and clinical practice guidelines,Reference Schull, Guttmann and Leaver 17 - Reference Skanes, Healey and Cairns 19 little work has been done on improving low ED anticoagulation rates. Given that less than one-fifth of patients were admitted, EPs were the key prescribers of appropriate antithrombotic (ASA) and anticoagulant medications for the majority of patients not already on these medications. It is discouraging that the majority of ED AFF patients are not correctly started on anticoagulation while in the community, and the reasons for this are likewise unclear. In 2014, almost 15% of Canadians lacked a family physician, 22 and many patients who do possess a family physician have difficulty accessing them, or specialist consultants, in a timely fashion.Reference Barua and Ren 23 , 24 Given this lack of follow-up care for some patients, 22 or potentially dangerous delays for others,Reference Barua and Ren 23 , 24 it is imperative that EPs provide optimal, evidence-based care in the ED. The prescribing of new anticoagulation is a key quality of care metric,Reference Schull, Guttmann and Leaver 17 - Reference Skanes, Healey and Cairns 19 and a recent multicentre Canadian study has shown that almost half of ED patients with AFF are discharged without appropriate anticoagulation.Reference Stiell, Clement and Rowe 25 Our study demonstrates that EPs can strive to optimize patient outcomes with evidence-based prescribing and care in the ED.
An important feature of our study is that we have identified two vulnerable patient groups for whom new anticoagulation at ED discharge is suboptimal. No women received new anticoagulation upon ED discharge prior to pathway implementation; after pathway implementation, the rate improved to 39.2%. (In comparison, after pathway implementation, 73.5% of eligible men were discharged with new anticoagulation from the EP.) Although the rates of appropriate anticoagulation for women are still dismal, and the reasons behind this are unknown, we believe that our pathway at least provides some improvement. In addition, our study suggests that older patients received lower rates of new anticoagulation upon ED discharge prior to pathway implementation. Our findings are similar to prior work that demonstrates gender and age-based differences in the anticoagulation of AFF patients, both in the EDReference Stiell, Clement and Rowe 25 and primary care settings.Reference Coll-Vinent, Martín and Malagón 26 , Reference Mashal, Katz and Shvartzman 27 , Reference Stafford and Singer 28 The discrepancies in our study were partially improved by our coordinated evidence-based intervention and further highlight its importance to optimize patient care and outcomes, although there is still room for improvement.
Prior ED-based AFF pathways have separately focused on control of rate or rhythm, LOS, and patient satisfaction. Decker et al. described an observation unit that reduced median LOS to 10.1 hours but did not report on anticoagulationReference Decker, Smars and Vaidyanathan 29 ; our LOS of 3.6 hours may compare favourably. Elmouchi et al. demonstrated that ED-based AFF pathways improve patient satisfaction and quality of life but did not measure new anticoagulation or ED LOS.Reference Elmouchi, VanOosterhout and Muthusamy 30 Although our study did not directly measure patient-centred AFF outcomes, a key finding of our study is that the 30-day ED revisit rate for CHF dropped from 13.2% to 2.3% following pathway implementation. It is unclear whether pathway implementation improved ED management of patients with AFF, or whether its presence resulted in the use of specific cognitive forcing strategies by EPs.Reference Croskerry 31 It is conceivable that patients would identify not having to return to the ED within 30 days of a sentinel visit for AFF as an important measure of quality of care. Further, despite our substantially decreased LOS, we did not observe increased rates of 30-day mortality or return to the ED within 30 days for AFF or serious complications thereof. This suggests that the development of a coordinated, evidence-based ED AFF pathway may not adversely affect patient outcomes and key performance metrics. However, prospective external validation of our findings is warranted.
LIMITATIONS
This study took place in two urban Canadian EDs with an AFF admission rate of approximately 20%, and it may be difficult to generalize to other settings.Reference McDonald, Pelletier and Ellinor 9 , Reference Cristoni, Tampieri and Mucci 32 - Reference Dankner, Shahar and Novikov 36 The lack of control sites means that the potential influence of outside influences upon these two centres is hard to determine. Importantly, this retrospective pre-post design is subject to lack of randomization and regression to the mean.Reference Harris, Bradham and Baumgarten 37 Although the study size is sufficient to estimate pathway effectiveness, the numbers may be too small to evaluate serious downstream adverse events. The ascertainment of individual comorbidities carries with it the potential for misclassification bias; this could affect each of the risk stratification scores, although the direction of bias would be unknown.
Our inclusion of 5 months of ED patients in our study to measure pathway outcomes ensures that we were unable to examine seasonal or secular trends, or regression to the statistical mean. We are also unable to comment on the sustainability of this intervention or extrapolation to other centres. We did not describe long-term follow-up, and it is unclear whether patients were adherent to EP recommendations and prescriptions.Reference Atzema, Austin and Chong 38 Furthermore, it is possible that such advice was either augmented or countermanded by a subsequent community physician. We did not assess either patient or provider preferences or satisfaction or the costs associated with our pathway. Finally, patients who had an out-of-region ED hospital admission or out-of-province death would not have been captured.
CONCLUSION
In conclusion, the implementation of an evidence-based, coordinated ED AFF pathway led to a significant improvement in the rate of patients with new appropriate anticoagulation upon discharge from the ED. A significant reduction in the mean ED LOS and ED revisit rates for CHF were observed, with no increase in patient mortality or key ED return rates.
Acknowledgements: The authors wish to thank Devon Webster, Rebecca Schonnop, Brian Kim, and Qadeem Salehmohammed for their assistance with data collection; and the St. Paul’s Hospital Emergency Associates for their support during this project. This work was previously presented at the Canadian Association of Emergency Physicians, June 2016, Quebec City, QC. Authors CD, JM, DK, BH, ST, and JR created and implemented the pathway in this study. DB, DH, RS, EG, and FS developed the protocol for this study and completed institutional REB submission. DB, CW, CV, and EG collected the data, and DB, CV, and FS conducted the analysis. All authors contributed to the preparation of the final manuscript.
Competing interests: None declared.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2017.418






