INTRODUCTION
Since the discovery of antimicrobials, the rate at which new effective antimicrobials have become available has slowed significantly,Reference Karras 1 , Reference Fishman 2 whereas broad prescribing patterns and ease of access have promoted growing rates of antimicrobial resistance and secondary antibiotic-associated infections. This trend has been epitomized by the development of organisms such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), extended spectrum beta-lactamases (ESBL), carbapenem-resistant Enterobacteriaceae (CRE), and Clostridium difficile (C. difficile).Reference Cohen 3 - Reference Baker, Acquisto and Ashley 8 As these trends have continued, patient morbidity, mortality, and health care system costs have grown.Reference Karras 1 , Reference Han, Kasahara and Edelstein 4 , Reference Harrison and Lederberg 5 , Reference May, Cosgrove and L’Archeveque 7 - Reference Gross, Morgan and Kinky 10
Antimicrobial stewardship was introduced to address these problems.Reference Shlaes, Gerding and John 9 Encouraging appropriate and judicious use of antibiotics reduces resistance rates at the local levelReference Fishman 2 , Reference Ohl and Dodds Ashley 11 , Reference Lipworth, Hyle and Fishman 12 and also improves patient outcomes.Reference Harrison and Lederberg 5 , Reference Shlaes, Gerding and John 9 In recent years, multiple studies have been published evaluating the effectiveness of comprehensive antimicrobial stewardship programs (ASPs) in reducing antimicrobial use in the inpatient setting.Reference Baker, Acquisto and Ashley 8 , Reference Ohl and Dodds Ashley 11 , Reference Lesprit, de Pontfarcy and Esposito-Farese 13 - Reference Lesprit, Landelle and Brun-Buisson 15 Unfortunately, prospective audit and feedback (PAAF), the default strategy used for most effective ASPs, is not feasible to implement in the emergency department (ED) due to high patient turnover and the need to provide emergent, around-the-clock care.
Several studies evaluating different ASP strategies in the ED have been published.Reference Baker, Acquisto and Ashley 8 , Reference Hecker, Fox and Son 16 - Reference Dumkow, Kenney and MacDonale 20 These include the use of phone callbacks for culture results returned after patient discharge,Reference Baker, Acquisto and Ashley 8 , Reference Randolph, Parker and Meyer 18 , Reference Dumkow, Kenney and MacDonale 20 standardized order sets, algorithms, pre-packaged antibiotic kits,Reference Hecker, Fox and Son 16 , Reference Ostrowsky, Sharma and DeFino 17 and clinician or patient educational interventions.Reference Metlay, Camargo and MacKenzie 19 In a call for further research into ED ASPs, May et al. outlined a comprehensive list of 11 different types of strategies that can be implemented in creating an ED ASP.Reference May, Cosgrove and L’Archeveque 7
Unfortunately, the unique environment of the ED presents several challenges to the successful implementation of an ASP. Introducing change into a complex health care environment requires an approach that accounts for health care workers’ knowledge, behaviors, and attitudes.Reference Cabana, Rand and Powis 21 The published literature suggests that barriers in the ED, including pressures related to volume, lack of patient follow-up, perceived pressure from colleagues or patients themselves to over-prescribe, and clinical practice inertia,Reference May, Gudger and Armstrong 6 , Reference May, Cosgrove and L’Archeveque 7 , Reference Dumkow, Kenney and MacDonale 20 are predominantly related to behaviors and attitudes. The most recently published guidelines on ASP implementation by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) specifically state that, “Qualitative assessments that can examine the impact of factors such as organizational culture [and] prescriber attitudes . . . are important to establish the context in which ASP implementation occurs.”Reference Barlam, Cosgrove and Abbo 22
Front-line ownership (FLO) is a methodology that has shown promise in addressing these challenges successfully.Reference Zimmerman, Reason and Rykert 23 , Reference Chironda, Clancy and Powis 24 It invites front-line staff to identify local challenges to program success and suggest locally viable solutions. Most published studies to date focused only on single top-down interventions such as adherence to guidelines or protocols rather than meaningful clinical outcomes such as changes in usage rates of antimicrobials. The FLO model provides the opportunity for “organic” development of a unique program to meet clinical objectives by designing multiple interventions through a bottom-up approach involving iterative communication between users familiar with the idiosyncrasies of the system in which they operate and those facilitating the program.
The focus of our study is to evaluate the change in antimicrobial utilization in the ED after implementation of an ASP using FLO principles and methodology.
METHODS
Study setting
Toronto East General Hospital is a community teaching hospital with a large urban ED servicing approximately 74,000 adult and pediatric patients annually from a diverse range of socioeconomic and cultural backgrounds.
Since April 2010, the hospital has had a successful inpatient ASP using PAAF as its primary intervention.
Prior to ASP initiation, the ED had a process to ensure follow-up on culture results. The ED receives a daily list of all positive culture results ordered by any ED physician. Each day one physician was designated to compare any positive culture results to the corresponding ED patient record. In the event that a patient had received no prescription or a prescription for an antibiotic to which the cultured bacteria was resistant, that physician became responsible for contacting the patient to arrange further care.
Intervention
A collaboration was initiated between the hospital inpatient ASP, administered by two of the hospital’s Infectious Diseases (ID) Division physicians, and the ED to improve and optimize antimicrobial utilization in the ED.
After the initial educational session outlining ASP principles, subsequent interventions were driven by the front-line ED physicians and facilitated by the ASP using FLO principles. The primary goal of this project was to optimize antimicrobial utilization. An additional strategy identified as critical to reaching our primary goals was to reduce unnecessary microbiology tests, specifically urine cultures. This is explained further in the following texts.
A schematic of interventions suggested and introduced by front-line staff over time is shown in Figure 1.
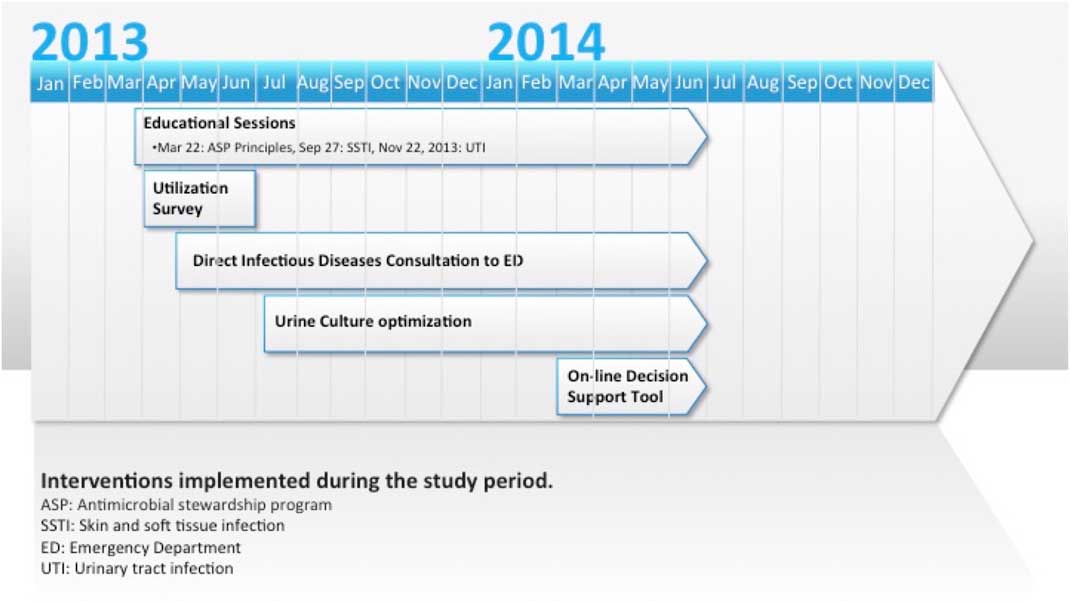
Figure 1 Emergency department antimicrobial stewardship interventions: 2012-2014.
The utilization assessment survey was provided to ED physicians through an online survey tool to determine their baseline antimicrobial utilization patterns and educational priorities (see Supplementary Appendix A). These priorities were the target for subsequent educational sessions, as shown in Figure 1. At the request of the ED physicians, these educational sessions focused on evidence-based treatment guidelines, created and delivered by ID specialists, based on local disease patterns for specific ID syndromes (i.e., skin and soft-tissue infections [SSTIs], urinary tract infections [UTIs]).
ED physicians identified the need for improved communication with an ID physician. Subsequently, the ID physician on-call was made available during regular work hours for consultation from the ED. ED physicians were encouraged to call him or her when uncertain for advice regarding antimicrobial utilization. The ID specialist on-call was drawn from a team of three physicians. The most common questions they received were related to complex SSTIs.
Due to a prolonged turnaround time from the laboratory in processing urinalyses, prior to the study intervention, nursing staff frequently collected urine samples from patients, sending them for both urinalysis and culture without physician orders, to ensure that results could be made available to the physician as soon as possible. Such culturing of urine even in the absence of any urinary symptoms or medical orders to do so was noted by the ED physicians to be a major driver of phone callbacks and later inappropriate antimicrobial prescribing for asymptomatic bacteriuria. As a result, an inter-professional group worked with front-line nursing staff to target a reduction in unnecessary culturing of urine for asymptomatic patients.
Last, a decision support tool was collaboratively developed with the ASP team to provide ED clinicians with rapid online access from any ED computer terminal to institution-specific antimicrobial suggestions for common ID presentations based on local susceptibility and resistance patterns as assessed by the inpatient ASP’s ID physicians (see Supplementary Appendix B). This decision support tool aimed to reduce use of antibiotics for which resistance was already prevalent (e.g., azithromycin monotherapy for community-acquired pneumonia, fluoroquinolones for UTIs), to avoid unnecessarily broad-spectrum antibiotics (e.g., carbapenems), to provide oral options for commonly used IV antibiotics (e.g., ceftriaxone), and to reduce those at high risk of triggering the C. difficile infection as a secondary infection (e.g., fluoroquinolones and clindamycin).
Members of the ASP were made available for ongoing discussion throughout the intervention period through emails and intermittent scheduled attendances at monthly ED physicians’ rounds. Feedback was also provided to the ED physician leadership quarterly on aggregate ED antimicrobial utilization and urine culture rates as part of a larger ASP quarterly report.
Methods of evaluation
To ensure comparability of the patient population before and after the intervention, ED patient visits and metrics, including Canadian Triage and Acuity Scale (CTAS) scores, average time to discharge, admission rates, and rates of return to the ED within 7 days, were extracted from the electronic medical records (EMRs) from the hospital decision support team after removal of data from patients who left without being seen by the ED physician.
Because the hospital EMR had no means of recording all outpatient discharge prescriptions nor of monitoring total days of therapy (DOT) for emergency room patients after discharge, antimicrobial utilization data were determined through financial charge data from within the ED and converted to defined daily doses (DDDs) using the World Health Organization standardized quotients. This strategy was in keeping with the most recent ASP guidelines from the IDSA and SHEA: “DOTs are preferred, but DDDs remain an alternative for sites that cannot obtain patient-level antibiotic use data.”Reference Barlam, Cosgrove and Abbo 22 DDD data were then standardized by 1,000 ED patient visits. March 2012 to March 2013 was defined as the pre-intervention period and April 2013 to June 2014 as the post-intervention period.
Data on the number of weekly urine cultures sent from the ED were provided by the microbiology lab and standardized by 100 ED patient visits. Data were available from the weeks starting on December 30, 2012 to June 23, 2014.
Analysis
Antimicrobial utilization data were first evaluated for the presence of seasonality using time series modeling. Because none of the best-fit models incorporated seasonality, this was not considered further in the analysis. Subsequently, generalized least squares were used to model a piecewise linear trajectory for each antimicrobial. Autocorrelation was assessed using Ljung–Box statistics. Because there was no evidence of autocorrelation, the linear piecewise models were used for hypothesis testing. Hypothesis testing consisted of an evaluation for stepwise changes in antimicrobial utilization to demonstrate initial effectiveness of the intervention and evaluation for differences in rates of change in antimicrobial utilization to assess for ongoing maintenance of change.
Weekly urine culture rates were evaluated using generalized least squares linear models incorporating the intervention and week of study. The models were compared using the analysis of variance (ANOVA), and the best-fit model utilizing an interaction between intervention and week of study was used for hypothesis testing.
A significance level of 0.05 was used for all analyses. All analyses were done using R version 3.1.2 (2011, Vienna, Austria) using the “forecast” and “Nlam” packages.
Ethics
As with any clinical intervention, the primary ethical concern was to ensure optimal patient care. All interventions were implemented to guide physician decision-making based on best available evidence without limiting the ability to adapt to individual patient needs or concerns. Ethics approval for this study was obtained through the Research Ethics Board at Toronto East General Hospital. No outside funding was received for this study nor were any conflicts of interest identified by anyone involved in implementing the interventions.
RESULTS
Comparison metrics from before and after the ASP intervention can be seen in Table 1. Responses to the antimicrobial utilization assessment survey can be found in Supplementary Appendix A.
Table 1 Emergency department (ED) patient volume and metrics from before and after introduction of the antimicrobial stewardship program (ASP)Footnote a
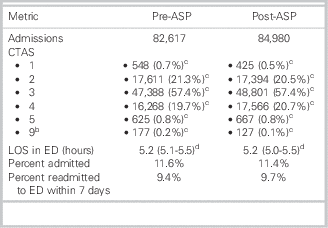
CTAS=Canadian Triage and Acuity Scale; LOS=length of stay.
a Excludes those who left without being seen by a physician.
b CTAS unknown.
c Total and percentage of total.
d Median and interquartile range.
Monthly antimicrobial utilization before and after initiation of the ASP is presented in Figure 2. At the start of the ASP intervention, there was a stepwise significant reduction in the use of azithromycin (p=0.006), ceftriaxone (p=0.045), ciprofloxacin (p=0.034), and moxifloxacin (p=0.008). Comparing the slopes of antimicrobial use before and after ASP implementation, there was a decreasing trend in the slope of use of all antimicrobials assessed outside of clindamycin and ertapenem (Table 2). There was a significant decrease after ASP implementation in the slope in use of ceftazidime (see Table 2) with a significant increase in the slope in use of clindamycin (see Table 2 and Figure 2). After the initial significant stepwise reduction in ceftriaxone and azithromycin use after ASP implementation, there was an increasing trend with a return to baseline use.

Figure 2 Monthly antimicrobial utilization expressed in defined daily doses (DDD) per 1,000 patient visits (PV) in the emergency department (ED) from March 2012 to June 2014. Note: Scatterplots of raw monthly utilization data are shown for each antibiotic from March 2012 to June 2014 with fitted linear model overlaying. The intervention initiation is shown as a break in the fitted model lines at April 2013. Pip-Tazo=piperacillin/tazobactam; sulfamethoxazole-tri=sulfamethoxazole-trimethoprim.
Table 2 Antimicrobial utilization in the emergency department (ED) before and after introduction of an antimicrobial stewardship program (ASP)
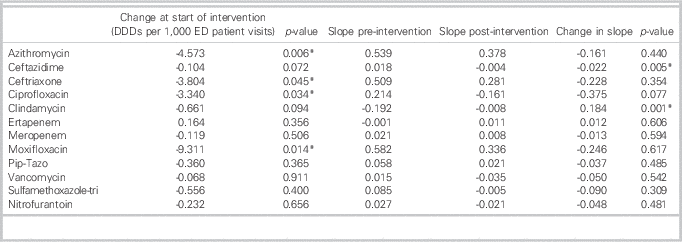
*Significant at a p value of<0.05.
DDDs=defined daily doses; Pip-Tazo=piperacillin/tazobactam; Sulfamethoxazole-tri=sulfamethoxazole-trimethoprim.
Weekly rates of urine cultures sent from the ED are presented in Figure 3. On July 15, 2013, after initiation of the intervention focused on reducing rates of urine cultures, there was a relatively abrupt drop in rates after a lag of approximately 3 weeks. The intervention was associated with a stepwise significant reduction in weekly rates of urine cultures (p<0.001) by 2.26 urine cultures per 100 ED patient visits. A statistical analysis did not show any significant difference in the slope of urine culture rates before and after the intervention (p=0.155).
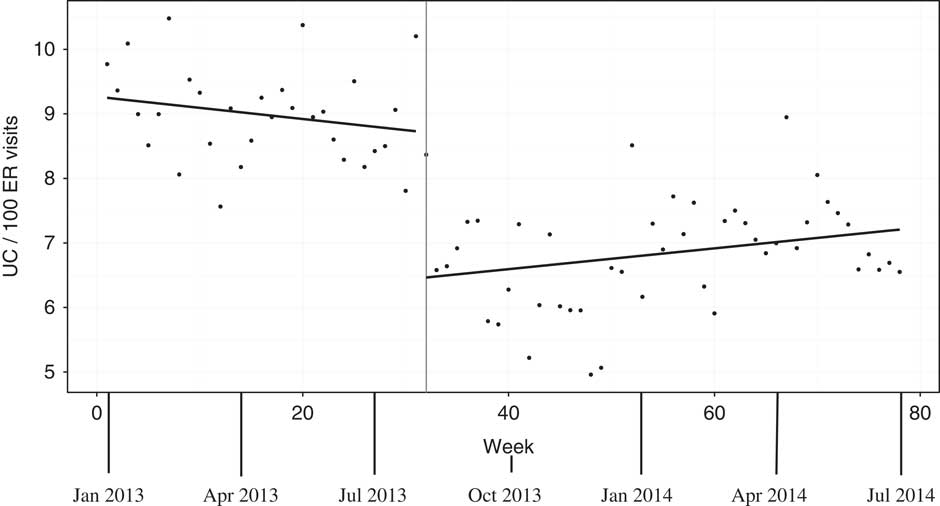
Figure 3 Weekly rates of urine cultures (UC) in the emergency department (ED) per 100 patient visits from December 30, 2012 to June 30, 2014. Note: Scatterplots of weekly UC rates are shown with fitted linear model overlaying. The intervention initiation is shown as a vertical line at the time of maximal intervention impact (week 32, about 3 weeks after the July 15, 2013 intervention).
DISCUSSION
Summary
Through an approach based on FLO and sequential implementation of multiple interventions, our ED showed a decrease in usage of antibiotics with high resistance rates (e.g., azithromycin, fluoroquinolones) and those at high risk of triggering C. difficile as a secondary infection (e.g., fluoroquinolones and clindamycin), and a sustained decrease in rates of urine cultures. Although this was a monocentric study, the use of a FLO approach allowed for the organic development of individualized solutions unique to the local ED environment.
Interpretation
Prior published ASP interventions in the ED have often been externally driven or organized in parallel to the work of the ED physicians themselves. By using a FLO approach, our study has shown a substantial decrease in unnecessary microbiology investigations and use of antimicrobials that could lead to adverse outcomes (e.g., resistance, ineffective prescribing, or secondary infection). The FLO approach allows for improved engagement of care providers and the development of novel solutions to challenges associated with improved antimicrobial utilization.
The flexibility of the FLO approach also leads to challenges regarding our traditional concept of reproducibility to other EDs. The FLO model emphasizes, “Creating local and context-appropriate solutions. . . . [It] does not yield solutions that can be copied from one setting where it worked, and then re-applied in the same way in a new setting and expected to work just as well. . . . Although the basic approach is consistent, the way in which it manifests will vary widely.”Reference Zimmerman, Reason and Rykert 23 Although the specific set of interventions used in our study may not work equally well in another ED, our experience with the FLO approach suggests that it may successfully lead other EDs to find their own unique interventions to optimize antimicrobial utilization and related investigations.
As a further point of interest, although the interventions in this study were developed using FLO methodology independently of the recently published IDSA and SHEA ASP guidelines, they are nevertheless entirely consistent with the recommendations made in them.Reference Barlam, Cosgrove and Abbo 22 FLO can be thought of as an engagement strategy, which may also help with the implementation of unique and also previously validated ASP interventions.
Antimicrobials are overused in the ED.Reference Karras 1 Prior published ASP interventions in the ED have focused on adherence to guidelines for specific infections or culture results rather than changes in antimicrobial utilization. By using a broader view of the ED as a whole, this study shows success through the lens of antimicrobial and culture utilization across our department. In the long term, further research may be helpful to see whether this change in department-wide antibiotic use can correlate to potential changes in the resistance pattern of bacteria in our geographic area.
Although outpatient and discharge prescriptions are not yet included in our data, recent efforts in our ED have been undertaken to maintain an online record of all discharge prescriptions from the ED. The incorporation of these data into future iterations of the ASP will provide an avenue to further improvement in prescribing patterns.
LIMITATIONS
The downside of the FLO multi-pronged approach to an ASP in the ED is that the contributions of each intervention to the overall outcome measures are confounded together. Thus, further studies elsewhere would be needed to isolate the relative effect of each intervention. Additionally, the generalizability of our intervention to other EDs is challenging to predict as a result of the flexibility of the FLO approach and the fact that this was a monocentric study. FLO may lead to a different set of ASP interventions than the ones we used if used in a different ED.
Because antimicrobial utilization data are presented in the form of DDD calculated from financial costs rather than DOT from medication administration records, it is impossible to be absolutely certain that the medication was actually administered to the patient. This limitation, however, is universal with all stewardship studies using DDD data. Additionally, the use of aggregate antimicrobial utilization data for the entire ED means that antimicrobials prescribed by non-ED specialists after referral from an ED physician, but prior to patient transfer from the department, were included in the data and may dilute the impact of our intervention on the ED physicians. Last, aggregate antimicrobial utilization data do not allow us to assess the appropriateness of antimicrobial therapy for individual patients.
Outpatient antimicrobial prescriptions are not captured by these data. Because only initial antimicrobial treatment in the ED prior to transfer or discharge was included, a significant proportion of ED prescribing practices is missed. Although outpatient data are not included, the changes seen in the pattern of antimicrobials used within the ED strongly suggest a cultural change in prescribing patterns within the ED induced by our front-line approach. The reduction in patterns of use for antimicrobials used during a patient’s ED stay may very well translate into improved outpatient patterns of use, yet further study would be required to confirm this.
Additionally, although the intervention appears effective across several antibiotics in generating an initial change in rates of use, the continuing upward slopes among several of these, commonly used antibiotics such as azithromycin, ceftriaxone, and moxifloxacin, suggest difficulties in sustaining reductions in antimicrobial use. Although ceftriaxone and azithromycin use returned to baseline levels, moxifloxacin use remained below baseline levels after intervention implementation.
Many of the ASP tools used in our study focused on educational interventions. Education often has its largest impact on quality improvement at the time that it is delivered with subsequent regression to the mean without reinforcement. Prospective audit and feedback, the most important antimicrobial stewardship tool among inpatient programs, is likely effective through its ability to consistently and repetitively educate providers on strategies to optimize antimicrobial use.Reference Fleming, Ali and Matelski 25 Although PAAF is not a feasible intervention in the ED, tools such as retrospective audit and feedback would be able to deliver the same repetitive educational opportunities. The impact of adding retrospective audit and feedback on the sustainability of initial decreases in antibiotic use seen with our intervention could be addressed by future studies.
CONCLUSIONS
The experience and results outlined in this article show the basis from which a broad multi-pronged ASP can be successfully implemented in the ED. Unlike previous studies that focused only on a single intervention, our study emphasizes the value of FLO and an iterative approach with multiple interventions. Regular physician engagement was a key component in creating the culture for a sustainable and successful ED ASP. Future studies would be helpful to evaluate the generalizability of this approach to ASP development in other EDs and to determine strategies to improve the sustainability of reductions in antimicrobial use.
Acknowledgements: The authors wish to thank the Emergency Department staff for their ongoing commitment to the project: Barley Chironda for his work in developing strategies to optimize urine cultures; Toronto East General Hospital Decision Support, especially Angela Chow, for their assistance with data requests; and John Matelski for his patience with the statistical analysis.
Financial support: This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests: None declared.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2017.11.







