CLINICIAN'S CAPSULE
What is known about the topic?
Emergency department physicians commonly manage acutely painful osteoporotic vertebral compression fractures resulting from minor trauma and seek nonopioid alternatives.
What did this study ask?
What evidence exists on the efficacy of calcitonin for managing acute pain associated with compression fractures?
What did this study find?
Calcitonin significantly reduced acute compression fracture pain (number needed to treat = 2) and improved function without significantly increasing the overall risk of side-effects.
Why does this study matter to clinicians?
Calcitonin appears to be an effective and safe alternative for the short-term management of acutely painful compression fractures.
INTRODUCTION
Compression fractures of the vertebrae represent a common type of osteoporotic fracture and are a common cause of emergency department (ED) visits and functional decline in the elderly.Reference Kim, Park and Park1,Reference Hopkins, Burke and Von Keyserlingk2 The lifetime risk of developing a painful compression fracture is 18% among females and 11% among males 60 years of age; however, many fractures are asymptomatic.Reference Nguyen, Ahlborg, Center, Eisman and Nguyen3 Acutely painful compression fractures tend to be precipitated by minor falls, bending, or twisting motions, and one case purportedly occurred while driving over a speed bump.Reference Aslan, Karcioglu, Katirci, Kandis, Ezirmik and Bilir4–Reference Cummings and Melton6 Compression fractures result in more acute care admissions in Canada than any other osteoporosis-related fracture type, except for hip and femur fractures.Reference Hopkins, Burke and Von Keyserlingk2 Moreover, compression fractures result in substantial health care usage and costs. In Canada, 36% of patients with ICD-10-CA codes for vertebral fracture attributable to osteoporosis were hospitalized within 1-year of their fracture, with a mean length of stay of 15 (standard error = 0.5) days.Reference Hopkins, Burke and Von Keyserlingk2 Acute pain associated with compression fractures ranges from mild to severe, and is treated with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids when additional pain relief is required. However, NSAIDs carry increased risks of gastrointestinal (GI) toxicity and renal insufficiency in older adults,Reference Smith7 and along with opioids are associated with adverse events related to drug interactions.Reference Budnitz, Lovegrove, Shehab and Richards8 In addition, their efficacy in treating pain associated with compression fractures has not been demonstrated.
Canadian clinical practice guidelines for osteoporosis suggest treating compression fracture-related pain with calcitonin; however, there is limited evidence to support this off-label use of the drug.Reference Papaioannou, Morin and Cheung9 Calcitonin is produced by C-cells of the thyroid and may reduce the transmission of pain signals by the central and peripheral nervous systems through increasing the secretion of beta-endorphins by neurons and decreasing cyclooxygenase, respectively.Reference Ito and Yoshimura10,Reference Felsenfeld and Levine11 There are multiple synthetic formulations of calcitonin; however, only salmon calcitonin is available in Canada. Data from new randomized controlled trials and trials missed previously may influence the findings reported in a prior systematic review on this topic by Knopp-Sihota et al. (2012) and their credibility.Reference Knopp-Sihota, Newburn-Cook, Homik, Cummings and Voaklander12 In addition, the effects of calcitonin on secondary outcomes, including function has not yet been evaluated.Reference Knopp-Sihota, Newburn-Cook, Homik, Cummings and Voaklander12,Reference Knopp, Diner, Blitz, Lyritis and Rowe13 Therefore, the objective of this systematic review is to systematically evaluate the efficacy of calcitonin in treating acute pain and evaluate its impacts on secondary outcomes, including length of hospital stay, ability to function, and quality of life.
METHODS
Study design and registration
We conducted this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1) (Figure 1). The study protocol was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO-CRD42018084850).Reference Moher, Liberati, Tetzlaff and Altman14
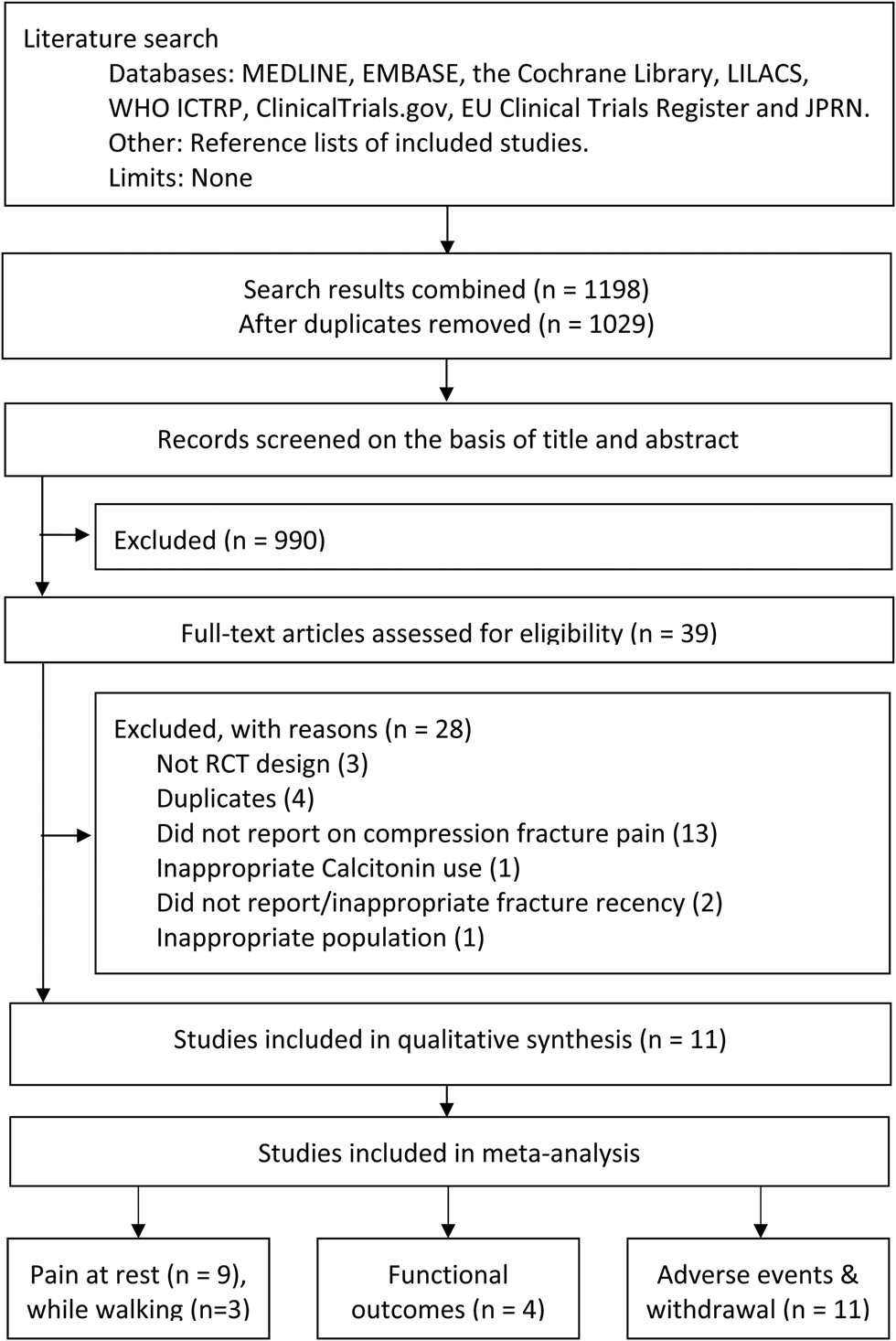
Figure 1. PRISMA flow diagram
Search strategy
MEDLINE, EMBASE, The Cochrane Library (Cochrane Database of Systematic Reviews), Cochrane Central Register of Controlled Trials, World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov, EU Clinical Trials Register, Latin American and Caribbean Health Sciences Literature Database, and Japan Primary Registries Network were searched without limits with assistance from a librarian (L.T.). Conference papers and reference lists of included studies were also searched. The search strategy was developed using Medical Subject Headings (MeSH) related to compression fracture, calcitonin, and pain (Supplemental Files).
Inclusion criteria
Randomized-controlled trials that enrolled older adults (mean >60 years of age) who suffered acute pain associated with a recent compression fracture (<16 weeks) were included. This definition was chosen because, while most clinical osteoporotic vertebral compression fractures result in the rapid onset of severe pain, some may present with multiple bouts of acute pain of lesser severity over a period of weeks to months.Reference Lyritis, Mayasis and Tsakalakos15 Studies that evaluated calcitonin (any route of administration, analogue, or dose) were considered for inclusion.
Data extraction and risk of bias assessment
Two reviewers (E.B., B.R.) extracted data and allocated bias using the Cochrane Collaboration's tool for risk of bias assessment independently and in duplicate using a standardized form. Trial investigators were contacted to obtain missing data. In cases where results were shown as graphs and original results were unavailable, data were extracted from figures using a Web-based tool.Reference Rohatji16 Disagreement between reviewers was resolved by a third reviewer (E.L.).
Statistical analysis
Included studies were grouped by the timing of outcome measures: 1 and 4 weeks. For pain and function scores, continuous outcomes reported as means with standard deviations were pooled for meta-analysis using a random-effects model to account for unexplained heterogeneity.Reference Higgins and Green17 Standard mean difference (SMD) and 95% confidence intervals (CIs) were calculated. To aid in the interpretation of the SMD, the probability of benefit (POB), which represents the probability that a person from the treatment group has a lower pain score than a person in the control group and number needed to treat (NNT) were determined as described by Kraemer and Kupfer.Reference Faraone18,Reference Kraemer and Kupfer19 For the analysis of adverse events and study withdrawal, dichotomous outcomes were pooled and risk ratios (RRs) with 95% CIs were calculated using the Mantel-Haenszel approach.Reference Higgins and Green17 For adverse events with five or more occurrences, p-values were computed with Fisher's exact test (two-tailed).
Assessment of heterogeneity
Between-study heterogeneity was assessed qualitatively and using I 2 and the χ2 test (>75% or <0.1, indicative of substantial heterogeneity, respectively).Reference Higgins and Green17 Subgroup analyses were performed to explore sources of heterogeneity. We decided a priori to conduct subgroup analyses for type of calcitonin.
p-values of < 0.05 were considered significant. All statistical analyses were performed in RevMan (version 5.3.5), except POB, NNT, and Fisher's exact test, which were calculated in RStudio (version 1.1.453) and GraphPad (version 8), respectively.
Grading of evidence
The certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. Risk of bias, inconsistency, indirectness, imprecision, and publication bias were considered.Reference Schünemann, Brożek, Guyatt and Oxman20
RESULTS
Our search yielded 1,198 records, of which 39 full-text articles were screened. Common reasons for the exclusion of records were related to study design and outcome, for example evaluating the efficacy of calcitonin for preventing of fractures instead of treating fracture pain. Eleven studies were included in the systematic review, with 713 participants.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Bordier, Julien and Caulin31 Two studies could not be pooled for meta-analysis of the primary outcome, pain at rest, because data were reported as median values and without a measure of varianceReference Lyritis, Ioannidis and Karachalios28,Reference Arinoviche, Arriagada and Jacobelli30 ; thus, nine studies were considered for meta-analysis of pain at rest with 641 participants, of whom 94.6% were female.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Pun and Chan29,Reference Bordier, Julien and Caulin31
Characterization of included studies
Study design and participants
Participants in included studies were generally between 60 and 70 years of age and presented with acute back pain attributed to compression fracture by clinical examination aloneReference Bordier, Julien and Caulin31 or in combination with X-ray and MRI imaging.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Endo, Fujino and Doi23–Reference Bordier, Julien and Caulin31 Fractures resulting from high-impact trauma, malignancies, and bone metabolism disorders were excluded; however, no included studies specified the mechanism of injury.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Bordier, Julien and Caulin31
Interventions and outcomes
Intervention groups received calcitonin as an intramuscular (IM)Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Lauro and Palmieri24,Reference Lyritis, Tsakalakos, Magiasis, Karachalios, Yiatzides and Tsekoura26,Reference Bordier, Julien and Caulin31 or subcutaneous (SC) injection,Reference Arinoviche, Arriagada and Jacobelli30,Reference Bordier, Julien and Caulin31 intravenous (IV) infusion,Reference Laroche, Cantogrel and Jamard25 intranasal spray (NAS)Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Pun and Chan29 or suppository.Reference Lyritis, Ioannidis and Karachalios28 Doses were 20 IU per week for elcatonin IM (synthetic eel calcitonin),Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Endo, Fujino and Doi23 50–100 IU per day for synthetic salmon calcitonin IM, SC, or NAS,Reference Lauro and Palmieri24,Reference Lyritis, Tsakalakos, Magiasis, Karachalios, Yiatzides and Tsekoura26–Reference Bordier, Julien and Caulin31 and 1.5 mg over a 4-hour IV infusion for synthetic human calcitonin.Reference Laroche, Cantogrel and Jamard25 Studies used a placeboReference Lyritis, Tsakalakos, Magiasis, Karachalios, Yiatzides and Tsekoura26–Reference Bordier, Julien and Caulin31 or active treatment of bisphosphonates/anti-osteoporosis drugsReference Tanaka, Yoshida, Kono and Ito22,Reference Lauro and Palmieri24,Reference Laroche, Cantogrel and Jamard25 or NSAIDsReference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Endo, Fujino and Doi23 in comparator groups. Nine studies permitted participants randomized to either group to take rescue analgesics (NSAIDs, acetaminophen, or unspecified).Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Endo, Fujino and Doi23,Reference Laroche, Cantogrel and Jamard25–Reference Arinoviche, Arriagada and Jacobelli30 Follow-up time ranged from 14 days to 6 months. All included studies scored pain using self-reported visual analogue scales.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Bordier, Julien and Caulin31 Five studies scored participant's self-reported function, including their ability to perform activities of daily living independently.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Endo, Fujino and Doi23–Reference Laroche, Cantogrel and Jamard25,Reference Arinoviche, Arriagada and Jacobelli30 Length of hospital stay, health-related quality of life, and compliance were not reported by any included studies (Supplemental Table S1).Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Bordier, Julien and Caulin31
Risk of bias
Risk of bias is summarized in Supplemental Figure S1. The majority of studies were double-blindReference Laroche, Cantogrel and Jamard25–Reference Bordier, Julien and Caulin31; however, results from open-labelReference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Endo, Fujino and Doi23 and single-blind designReference Lauro and Palmieri24 studies may be affected by performance and/or detection bias. Randomization and allocation concealment were specified in threeReference Endo, Fujino and Doi23,Reference Lauro and Palmieri24,Reference Pun and Chan29 and two,Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Tanaka, Yoshida, Kono and Ito22 trials, respectively. Selective reporting and incomplete reporting of outcome data were uncommon.Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Lyritis, Ioannidis and Karachalios28
Analgesic efficacy of calcitonin
All included studies reported that calcitonin significantly reduced acute pain associated with recent compression fractures.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Bordier, Julien and Caulin31 Data were grouped based on type of calcitonin and dose due to the presence of heterogeneity (I 2 = 90% at 1 week and 93% at 4 weeks; Supplemental Figure S2). Salmon calcitonin improved pain scores at 1 week (SMD, -1.54; 95% CI, -2.02 – -1.06) (Figure 2) with a high certainty of evidence as assessed with GRADE criteria.Reference Lauro and Palmieri24,Reference Lyritis, Tsakalakos, Magiasis, Karachalios, Yiatzides and Tsekoura26,Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Pun and Chan29 The POB for salmon calcitonin at 1 week was 86.2% (95% CI, 77.3–92.3%), representing a NNT of 2 (95% CI, 2–2). At 4 weeks, a high level of heterogeneity was present (I 2 = 95%); thus, the data were not pooled (Figure 3).Reference Lauro and Palmieri24,Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Pun and Chan29,Reference Bordier, Julien and Caulin31 Weekly elcatonin modestly improved pain scores at 4 weeks (SMD, -0.41; 95% CI, -0.62 – -0.20; Figure 3),Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Endo, Fujino and Doi23 but not 1 week (SMD, -0.09; 95% CI, -0.42–0.25; Figure 2).Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Tanaka, Yoshida, Kono and Ito22 The certainty of evidence was downgraded to moderate due to concerns about detection and performance bias (Table 1).
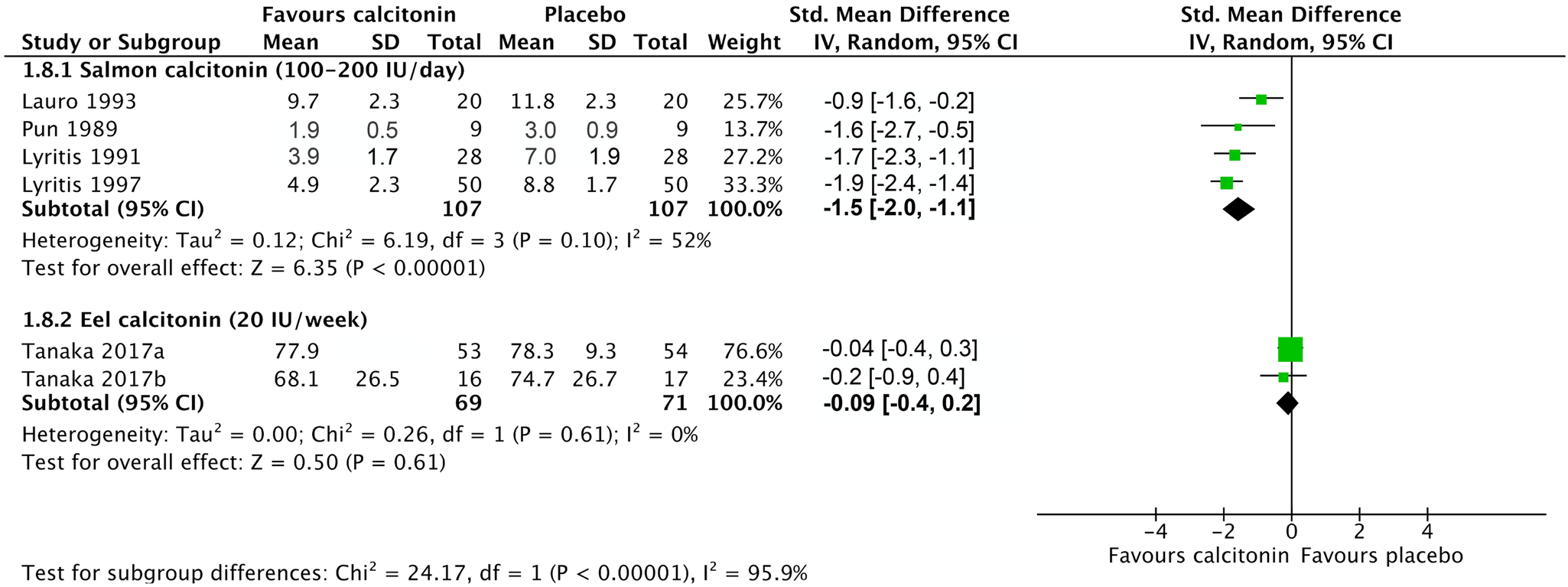
Figure 2. The effects of elcatonin, salmon, and synthetic human calcitonin on pain associated with compression fracture after 1 week of treatment

Figure 3. The effects of elcatonin, salmon, and synthetic human calcitonin on pain associated with compression fracture after 4 weeks of treatment
Table 1. Summary of findings table for key outcomes including assessment with GRADE

Note: The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
*Trials have small n (n < 100).
†No prospective protocols were available.
‡Studies were unblinded or single-blinded.
§Substantial unexplained heterogeneity.
¶Wide CIs.
#Assessed at a range of time points (1–6 months).
Effects of calcitonin on function
At 1 week, function was improved after daily treatment with salmon calcitonin in one study; however, weekly treatment with elcatonin did not (data not pooled due to heterogeneity, I 2 = 78%).Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Lauro and Palmieri24 At 4 weeks, all types of calcitonin improved function (SMD, -0.48; 95% CI, -0.79 – -0.17)Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21,Reference Endo, Fujino and Doi23–Reference Laroche, Cantogrel and Jamard25 with a POB of 63.3% (95% CI, 71.2–54.8%) and NNT, 4 (95% CI, 3–11). The level of certainty for both time points was low.
Adverse events and study withdrawal
Seven hundred thirteen patients across 11 studies had a nonsignificantly higher risk of developing adverse events (RR, 2.10; 95% CI, 0.87–5.08; I 2 = 47%) with low certainty (Figure 4). The certainty of evidence was downgraded due to imprecision and concerns about bias introduced by open-label and single-blind studies. Nausea, vomiting, and enteric side effects were reported by 22 participants in calcitonin treatment groups, but were not significantly increased compared with the control group (p = 0.511).Reference Endo, Fujino and Doi23,Reference Lauro and Palmieri24,Reference Lyritis, Tsakalakos, Magiasis, Karachalios, Yiatzides and Tsekoura26,Reference Lyritis, Ioannidis and Karachalios28,Reference Arinoviche, Arriagada and Jacobelli30 Eleven participants reported mild or nonspecific side effects, including hot flushes, redness, and injection site pain (Table 2).Reference Endo, Fujino and Doi23,Reference Laroche, Cantogrel and Jamard25–Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Arinoviche, Arriagada and Jacobelli30 Twelve participants withdrew from four studies due to a desire for stronger analgesia (n = 6 in placebo groups), erythema (n = 3), enteric disturbances (n = 3), or unspecified reasons (n = 2).Reference Endo, Fujino and Doi23,Reference Laroche, Cantogrel and Jamard25,Reference Lyritis, Ioannidis and Karachalios28,Reference Arinoviche, Arriagada and Jacobelli30 Five participants were lost to follow-up.Reference Endo, Fujino and Doi23

Figure 4. Relative risk of adverse events associated with calcitonin use to treat pain associated with compression fracture
Table 2. Adverse events

Interpretation
This systematic review synthesizes the findings of 11 randomized-controls trials investigating the efficacy of calcitonin for treating acute pain associated with compression fractures. High quality evidence was found supporting the efficacy of salmon calcitonin for reducing compression fracture pain after 1 week of treatment, which is consistent with previous studies.Reference Knopp, Diner, Blitz, Lyritis and Rowe13,Reference Moher, Liberati, Tetzlaff and Altman14 The narrative synthesis suggests salmon calcitonin may reduce pain at later time points; however, data could not be pooled due to substantial heterogeneity between studies. Low certainty evidence showed that low-dose elcatonin improved pain after 4 weeks, but not 1 week. The effect of calcitonin on function has not been reported in previous reviews, but is relevant due to its association with ED visits in the elderly.Reference Laudisio, Marzetti, Franceschi, Bernabei and Zuccala32 Salmon calcitonin and elcatonin were found to improve function over longer time periods with low certainty evidence. There was low certainty evidence that calcitonin does not increase the risk of adverse events. Adverse events reported in included studies were similar to those described for other indications of calcitonin.
No studies have directly compared the efficacy of salmon calcitonin against elcatonin, which is used in Japan, but is not approved by Health Canada.Reference Endo, Fujino and Doi23,33 It is unclear if the lack of efficacy of elcatonin is related to the low dose used (20 IU per week) relative to studies evaluating salmon or synthetic human calcitonin. It is possible that the effect between salmon calcitonin and elcatonin may be confounded by other factors, such as route of administration. A double-blind equivalence study comparing SC and NAS calcitonin formations for the relief of acute pain resulting from compression fractures found there was no difference in effect based on the route of administration.Reference Combe, Cohen and Aubin34 Thus, these potential confounders are unlikely to account for this finding.
The results of our study indicate that salmon calcitonin may be an appropriate adjunct treatment for reducing pain in elderly patients with compression fractures and no new neurologic injury who have received the maximum dose of acetaminophen. NSAIDs and other narcotics are commonly contraindicated in older adults with comorbid conditionsReference Frank and Weir35; therefore, calcitonin is a promising therapy for pain reduction. In addition, the adverse events reported with calcitonin use appeared mild and may be superior to those associated with NSAIDs and opioids in elderly persons.Reference Frank and Weir35 Treatment with 50–200 IU IM or NAS salmon calcitonin, daily for 1 week was effective at reducing pain.Reference Lauro and Palmieri24,Reference Lyritis, Tsakalakos, Magiasis, Karachalios, Yiatzides and Tsekoura26,Reference Lyritis, Paspati, Karachalios, Ioakimidis, Skarantavos and Lyritis27,Reference Pun and Chan29 In Canada, 1 U of calcitonin (200 IU for injection) costs of $30.48.36
The long-term use of calcitonin nasal sprays appears to be associated with a slight increase in cancer risk,37,Reference Wells, Chernoff, Gilligan and Krause38 although none of the included studies reported cancer as an adverse event.Reference Tanaka, Yoshida, Kono, Oguma, Hasegawa and Ito21–Reference Bordier, Julien and Caulin31 Health Canada withdrew nasal salmon calcitonin, because it no longer has a favorable risk–benefit profile for treating osteoporosis and recommended that the use of injectable calcitonin be limited to less than 3 months.37 Further research is required to clarify the safety of short-term use of calcitonin. However, the results of this study suggest that the short-term use of calcitonin may still be beneficial for treating acute pain associated with compression fractures in older adults. Calcitonin may be initiated by the emergency physician and continued by the patient's family doctor if appropriate. The decision to use calcitonin should reflect the patient's values and preferences, because pain relief may be prioritized over a small increase in cancer risk by some individuals.
LIMITATIONS
We followed rigorous protocol (PRISMA), prospectively registered our study and conducted a comprehensive search of electronic databases, clinical trials registries and grey literature. A random effects model, which tends to produce more conservative effect-sizes, was used and the inclusion of extremely small studies did not appear to artificially increase the effect size. The decision to conduct a subgroup analysis based on the type of calcitonin used was made a priori. Consequently, the pooled effect size was derived from a small number of trials and may have limited generalizability. The small sizes of included trials might have contributed to imprecise effect sizes. We were unable to assess outcomes based on pain severity through subgroup analyses, because all studies reported moderate-to-severe pain at baseline. Future studies should evaluate outcomes based on pain severity, because these results may underestimate the beneficial effects of calcitonin for mild-to-moderate pain. Osteoporotic patients were identified based on clinical exam and X-ray or MRI interpretation in all included studies except one,Reference Bordier, Julien and Caulin31 which are subjective and might have contributed to heterogeneity. Finally, although our results were obtained from a comprehensive review of the published literature, there is the potential for unpublished negative trials to introduce bias. Future randomized-trials must strengthen the base of evidence for calcitonin as an analgesic.
CONCLUSIONS
Short-term use of salmon calcitonin, but not low-dose elcatonin, appeared to reduce acute pain associated with compression fracture in older adults without significantly increasing the risk of adverse events. Both types of calcitonin may improve function after compression fractures. Calcitonin may be considered as an alternative to opioid and nonopioid analgesic in older adults with compression fractures in emergency and primary care settings, but should be used on a short-term basis only due limitations in the evidence.
Supplemental material
The supplemental material for this article can be found at https://doi.org/10.1017/cem.2019.490
Acknowledgments
We thank Ms. Lorraine Toews from the Health Sciences Library at the University of Calgary for her assistance in developing and piloting search strategies and Dr. Jennifer Knopp-Sihota for her advice in developing the project.
Financial support
This work was supported by the Emergency Medicine Strategic Clinical Network Summer Studentship grant (E.B.).
Protocol registration
PROSPERO-(CRD42018084850)
Competing interests
The authors (B.R., E.B., and E.L.) do not have any conflicts of interest relevant to this manuscript.








