INTRODUCTION
Resuscitation-related injuries have been identified and treated since chest compressions were introduced into clinical practice in 1960, but their reported frequencies have varied greatly.Reference Krischer, Fine and Davis 1 Previous autopsy studies have analysed the incidence, identified predisposing factors including sex, and characterized the anatomic location of resuscitation-related injuries.Reference Krischer, Fine and Davis 1 - Reference Black, Busuttil and Robertson 4 Recent reports have focused on cardiopulmonary resuscitation (CPR) duration and depth, and identified injuries using chest radiography, computed tomography (CT), and bedside ultrasonography in both survivors and non-survivors.Reference Kim, Yang and Sung 5 - Reference Hellevuo, Sainio and Nevalainen 7 Because the methods for identifying injuries and definitions of severe injuries have varied so considerably, the reported incidence of significant injuries has varied from 0.2% to 10%.Reference Krischer, Fine and Davis 1 , Reference Hoke and Chamberlain 3 , Reference Kralj, Podbregar and Kejzar 8 , Reference Miller, Rosati and Suffredini 9 Lethal resuscitation-related injuries can induce hemodynamic instability and recurrent arrest, even after the original cause of arrest is corrected.
Because extracorporeal cardiopulmonary resuscitation (ECPR) restores perfusion to vital organs such as brain and injured myocardium, the window of time available for the correction of reversible etiology can be extended.Reference Nagao, Kikushima and Watanabe 10 - Reference Lee and Hong 12 ECPR thus works as a bridge until effective cardiac output is recovered.
Here, we describe two cases of cardiac rupture after CPR that demonstrated recurrent cardiovascular arrest with pulseless electrical activity (PEA) after the correction of presumed cardiac etiology of the original arrest. The cardiac rupture was identified under extracorporeal cardiopulmonary life support and repaired via open thoracotomy.
CASE 1
A 60-year-old woman with hypertension and vasospastic angina who had undergone coronary artery bypass grafting in 2001 was admitted to a local clinic for fiberoptic gastroscopy. Cardiac arrest occurred shortly after insertion of the gastroscope, and CPR was begun by a physician. The initial rhythm was asystole at the time of arrival of the paramedics. Twenty-eight minutes elapsed between the time of the arrest and arrival at the emergency department (ED). The vital signs on arrival at ED were blood pressure of 85/70 mm Hg, heart rate of 131 beats/min. She was intubated, was comatose, and her body temperature was 35.4°C. Cardiac arrest with PEA recurred several times. ECPR (Capiox Emergency Bypass System, Terumo Inc., Tokyo, Japan) was begun 82 minutes after ED arrival due to recurrent arrest, despite the use of inotropic agents and vasopressors. Coronary angiography and balloon angioplasty were performed at the ostial occlusion lesion of the obtuse marginal artery (Figure 1, A and B). Several medications were administered: 300 mg aspirin, 600 mg clopidogrel, and 6000 IU heparin. The patient showed ventricular fibrillation (VF) and PEA after angioplasty, and extracorporeal cardiopulmonary membrane oxygenation (ECMO) was maintained. After coronary angiography, echocardiography revealed cardiac tamponade, and pericardiocentesis removed 250 mL of nonclotting blood. As refractory shock persisted in spite of pericardiocentesis, open thoracotomy was performed 4 hours after ED arrival (see Figure 1, C and D). A large pericardial hematoma and enlarged edematous heart were identified, and a 2- to 3-cm vertical laceration of the left ventricle on the lateral free wall was repaired. A second open thoracotomy was performed due to low cardiac output and a massive left hemothorax on the second hospital day (HD). The sternum was left open because of the enlarged, edematous heart, and closure of the sternum and weaning from ECMO occurred on HD 6. The patient had a clear sensorium after discontinuing sedatives on HD 6 but developed left lower extremity motor weakness on HD 17. Diffusion-weighted magnetic resonance imaging revealed an embolic infarction in the right upper basal ganglia and the genu of corpus callosum. The patient was ultimately transferred to a rehabilitation centre for stroke rehabilitation with a Glasgow Coma Scale (GCS) score of 15 on transfer.
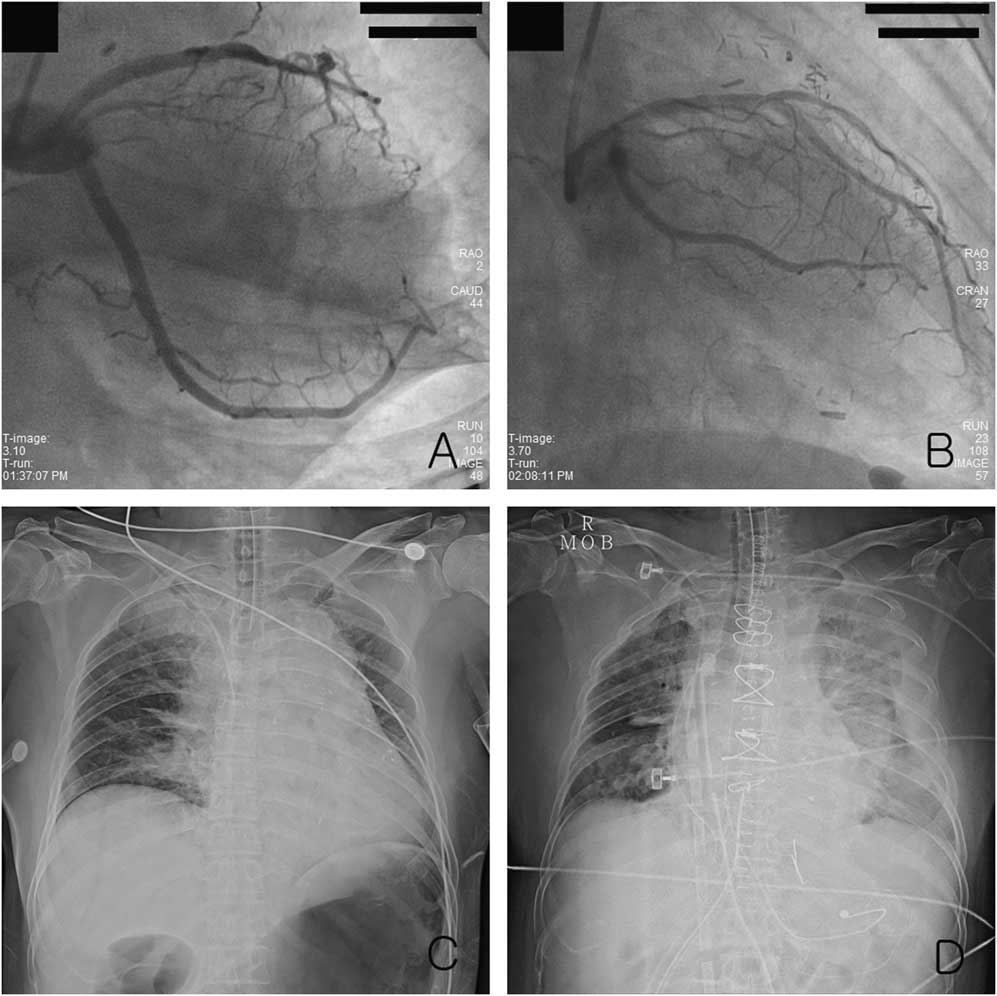
Figure 1 The right anterior oblique view of coronary angiography: A) showed the occlusion of the obtuse marginal artery and B) revealed that coronary arterial flow after balloon angioplasty was improved. Chest radiography showed C) the wide mediastinum before thoracotomy and D) pulmonary edema with pleural effusion after thoracotomy.
CASE 2
A 68-year-old woman with hypertension was admitted to the hospital for surgery to treat early-stage gastric cancer. The patient complained of diaphoresis while on the surgical ward on the morning of a planned discharge on the 8th postoperative day, and then experienced a cardiac arrest. Her initial rhythm was PEA, and this persisted despite advanced cardiac life support. The resuscitation team presumed the arrest to be of cardiac origin and activated the ECMO team. ECMO (Capiox Emergency Bypass System, Terumo Inc, Tokyo, Japan) was implemented during CPR at 51 minutes after arrest. After administration of inotropic agents and ECMO, the blood pressure was 70/40 mm Hg. Coronary angiography revealed total occlusion of the right coronary artery, and percutaneous coronary intervention (PCI) was performed (Figure 2, A and B). The following medications were administered: 300 mg aspirin, 600 mg clopidogrel, and 6000 IU heparin. Afterwards, echocardiography showed a small left ventricle with severe diffuse hypokinesia and ventricular systolic dysfunction. Despite maximal dosing of vasopressors, inotropic agents, and massive transfusion, hemodynamic instability persisted. Subcutaneous emphysema and pleural effusion were identified radiographically (see Figure 2, C). A left-sided tube thoracostomy was performed 3 hours after arrest, and 3660 mL of non-clotting blood drained over the next 6 hours. The patient underwent posterolateral thoracostomy 10 hours after arrest. A pericardial rupture near the pulmonary artery and a hematoma inside the thoracic cavity were observed. The patient showed refractory hemodynamic instability with a right-sided hemothorax on HD 2 (see Figure 2, D). Tube thoracostomy drained 780 mL of blood. The patient showed organ failure, coagulopathy, and refractory shock with severe ventricular dysfunction, despite ECMO, and died on HD 2.
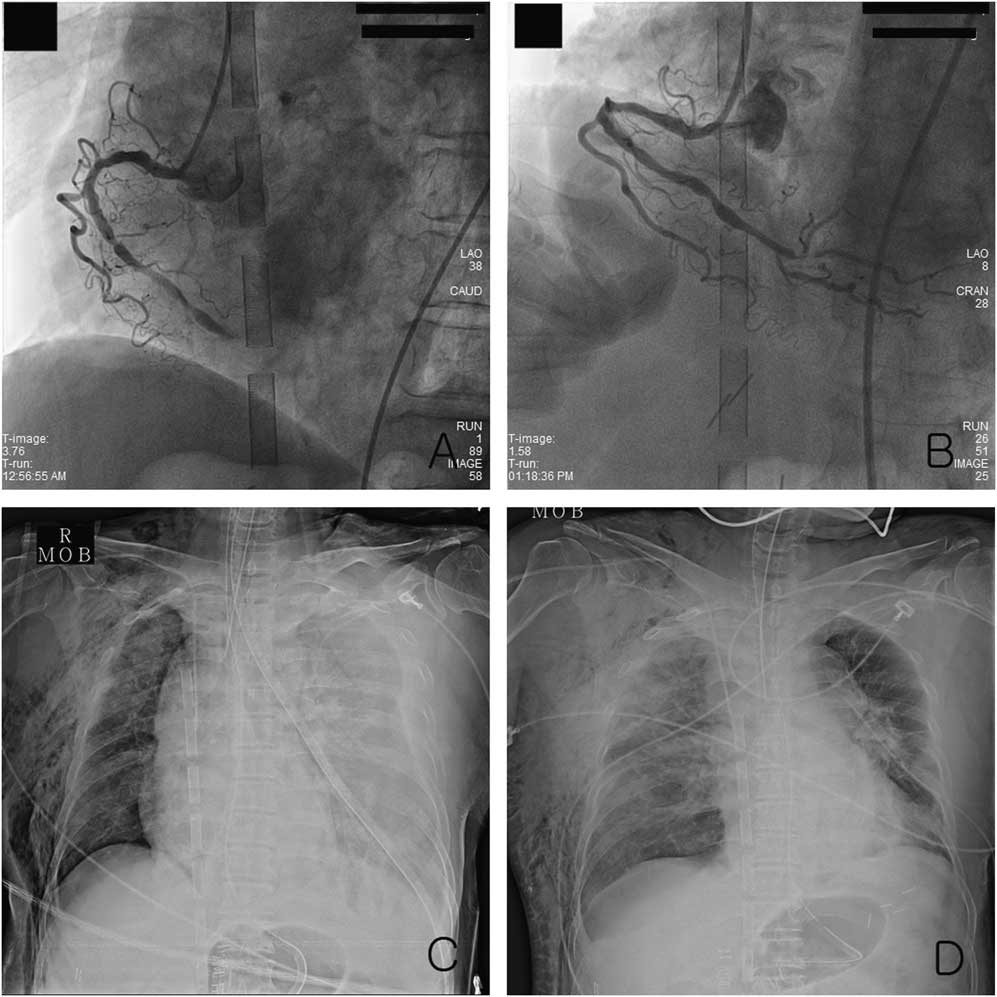
Figure 2 The left anterior oblique view of coronary angiography: A) revealed total occlusion of the right coronary artery and B) improved arterial flow after percutaneous coronary intervention. Chest radiography showed C) a subcutaneous emphysema and left-sided pleural effusion before thoracotomy and D) a right-sided hemothorax and pulmonary edema on the 2nd hospital day after thoracotomy.
DISCUSSION
We describe two patients with persistent PEA, recurrent arrest, and a total CPR duration <60 minutes (47 minutes and 51 minutes, respectively) before ECPR implementation. The primary cause of arrest in both cases was presumed to be myocardial infarction, and the patients were treated with angioplasty and PCI with ECPR. Although antithrombotic and anticoagulant drugs were administered, coagulation test results were within therapeutic range. Unexplained hemodynamic instabilities with hemothorax and pericardial effusion were considered after presumed etiology was corrected. As ECPR extended the effective resuscitation window, cardiac injuries could be detected, and open thoracotomy was performed under ECPR.
The most common complications of chest compression during resuscitation are relatively minor thoracic wall injuries, such as fractures of the rib and sternum. More serious injuries to the cardiovascular and pulmonary systems may cause iatrogenic, but potentially reversible causes of, resuscitation failure.Reference Krischer, Fine and Davis 1 , Reference Lederer, Mair and Rabl 2 , Reference Kim, Park and Kim 6 , Reference Kralj, Podbregar and Kejzar 8 , Reference Miller, Rosati and Suffredini 9 The incidence of such injuries has varied in the literature,Reference Lederer, Mair and Rabl 2 , Reference Hoke and Chamberlain 3 , Reference Kim, Yang and Sung 5 , Reference Kim, Park and Kim 6 , Reference Kralj, Podbregar and Kejzar 8 , Reference Miller, Rosati and Suffredini 9 in part due to variations in definitions of severe injuries.Reference Krischer, Fine and Davis 1 - Reference Hoke and Chamberlain 3 , Reference Kralj, Podbregar and Kejzar 8 , Reference Miller, Rosati and Suffredini 9 Several reports suggest that CPR-related skeletal chest injuries occur at a rate of 13% to 97% and severe injuries at a rate of 0.5% to 1.85%.Reference Krischer, Fine and Davis 1 - Reference Hoke and Chamberlain 3 , Reference Kralj, Podbregar and Kejzar 8 A systematic review by Miller et al. reported a rate for manual CPR-associated injuries of 32% to 45% and for major injuries of 7%.Reference Miller, Rosati and Suffredini 9 It is difficult to estimate the lethality of resuscitation-related injuries, given the limited success of conventional cardiopulmonary resuscitation (CCPR). Indeed, the incidence of life-threatening injuries has almost certainly been underestimated, especially when based on survivors only. Plain chest radiography and external examination can miss injuries in both survivors and non-survivors.Reference Lederer, Mair and Rabl 2 , Reference Kim, Yang and Sung 5 , Reference Kim, Park and Kim 6 , Reference Corbett and O’Callaghan 13 Recently, bedside sonography during CPR may help detect potentially lethal injuries.
Stiell et al. found that maximum survival rate was shown in the depth interval of 40.3 to 55 mm, although increased compression depth is strongly associated with better survival.Reference Stiell, Brown and Nichol 14 Hellevuo et al. reported that deeper chest compression (>6 cm) was associated with more resuscitation-related injuries. Their study showed that mean and peak compression depth and peak force were increased, especially after the application of 2010 American Heart Association (AHA) guidelines.Reference Hellevuo, Sainio and Nevalainen 7 The 2015 AHA guidelines also recommend chest compressions to a depth of at least 5 to 6 cm and a compression rate of 100 to 120/min. for improved survival and fewer injuries.Reference Neumar, Shuster and Callaway 15 , Reference Kleinman, Brennan and Goldberger 16
The effect of CPR duration on the incidence of injuries is unclear.Reference Krischer, Fine and Davis 1 , Reference Hoke and Chamberlain 3 , Reference Black, Busuttil and Robertson 4 , Reference Kim, Park and Kim 6 Several reports suggest that damage may be more likely to occur in early stage of CPR, because weaker compression due to increased fatigue in prolonged CPR would be associated with fewer complications.Reference Hoke and Chamberlain 3 , Reference Baubin, Rabl and Pfeiffer 17 , Reference Baubin, Sumann and Rabl 18
Although it is difficult to define risk factors for injuries due to the variability in the results on resuscitation-related injuries, several studies have reported that older patients due to degenerative skeletal changes, women due to osteoporosis, compression depth deeper than 6 cm and active compression-decompression mechanical device showed a higher injury rate.Reference Lederer, Mair and Rabl 2 - Reference Kim, Park and Kim 6 , Reference Miller, Rosati and Suffredini 9 , Reference Baubin, Rabl and Pfeiffer 17 - Reference Smekal, Johansson and Huzevka 19 The two cases reported here occurred in older women without mechanical compression devices, but the peak compression depth was unknown. Both bedside ultrasonography and plain chest radiography were used to detect injuries in our cases.
As ECPR provides adequate oxygenated blood delivery to sustain organs as a bridge for recovery, the effective resuscitation time for identifying and correcting etiology of arrest can be increased during ECPR. Its application can afford opportunity to detect and manage severe resuscitation-related injuries, which could be the secondary cause of arrest, even after correction of primary etiologies. Recent guidelines recommend that ECPR may be considered for selected patients with the suspected potentially reversible etiologies (myocardial infarction, massive pulmonary embolism, malignant arrhythmia, and so on) of the cardiac arrest during a limited period of mechanical cardiorespiratory support when rapidly available.Reference Brooks, Anderson and Bruder 20 Most reports about ECPR involved patients ages 18 to 75 years, with a presumed cardiac etiology of arrest, after CCPR for more than 10 minutes fails to restore spontaneous circulation.Reference Maekawa, Tanno and Hase 11 , Reference Kim, Kim and Lee 21 - Reference Wang, Chen and Ma 25 Kim et al. reported good outcomes in witnessed arrest without asystole as initial arrest rhythm.Reference Shin, Choi and Jo 23 Maekawa et al. suggested an implementation target of ECPR within 60 minutes of the start of CPR predicted favorable neurologic outcome.Reference Maekawa, Tanno and Hase 11 Of course, ECPR is a highly invasive procedure that requires considerable resources, expertise, a well-coordinated health care system, and raises ethical concerns. According to the meta-analysis of Kim et al., ECPR may give a chance for better survival and neurologic outcome than CCPR, especially at 3 to 6 months, although the survival benefit of ECPR with out-of-hospital cardiac arrest was not clearly shown.Reference Kim, Kim and Lee 21 Despite uncertain indications, ECPR may offer survival and better neurological outcomes than CCPR for selected candidates unresponsive to CCPR.Reference Maekawa, Tanno and Hase 11 , Reference Kim, Kim and Lee 21 - Reference Wang, Chen and Ma 25
Although ECPR should not be a routine alternative to conventional advanced cardiac life support, it should be considered selectively, based on hospital and patient factors. In addition to these considerations, ECPR may allow for the identification and correction of secondary injuries related to the initial resuscitation efforts, which should be considered as another correctable cause of refractory arrest.
CONCLUSION
Because resuscitation-related injuries can be time-dependent, reversible causes of recurrent arrest, it is necessary to evaluate injuries rapidly as correctable etiologies of arrest. ECPR may be considered for selected patients with potentially reversible etiology of the cardiac arrest in setting where it can be rapidly implemented. The early application of ECPR may provide opportunities for identifying and correcting lethal CPR-associated complications, as well as identifying arrest etiologies. It may be necessary to consider injuries as etiologies, especially in an older female patient experiencing recurrent arrest, or when chest compression depth exceeds 6 cm as 2015 AHA recommendations.
Competing interests: None declared.




