CLINICIAN'S CAPSULE
What is known about the topic?
Opportunities to initiate buprenorphine/naloxone and facilitate addictions referrals are often missed for emergency department (ED) patients presenting in opioid withdrawal.
What did this study ask?
In an ED program offering buprenorphine/naloxone and community-based addictions follow-up for opioid use disorder, what are six-month retention rates?
What did this study find?
Approximately 88% of patients consented to ED-initiated treatment/referral, and 37% continued treatment six months after their initial visit.
Why does this study matter to clinicians?
Local implementation of a similar program using existing community resources could lead to better care for patients presenting with opioid use disorder.
INTRODUCTION
Canada is amid an opioid crisis, with unintentional drug overdoses becoming a public health crisis. It is estimated that there were 3,998 apparent opioid-related deaths in Canada in 2017, with no regions spared: 76% of opioid-related deaths occurred in males, 27% in individuals between 30 and 39 years of age, and an increasing amount of opioid-related deaths (72%) involved fentanyl.Reference Gomes1,2 The pervasiveness of the opioid crisis is highlighted by the fact that opioid-related deaths are increasing in all age groups, both sexes, and all income brackets.Reference Gomes1
Based on recent Canadian guidelines, buprenorphine/naloxone is considered first-line for opioid use disorder (OUD) to reduce the risk of toxicity, morbidity, and mortality.Reference Bruneau, Ahamad and Goyer3 Compared with methadone, buprenorphine/naloxone has a milder adverse effect profile, requires a shorter time (1–3 days) to achieve therapeutic dose, and has a lower risk of toxicity and drug interactions.Reference Bruneau, Ahamad and Goyer3 Both buprenorphine/naloxone and methadone treatments are associated with reduced all-cause mortality and should be considered for all patients with OUD.Reference Larochelle, Bernson and Land4 Previous data have shown that only 10.8% of patients with substance use disorder receive specialty treatment annually.Reference Bogenschutz, Donovan and Mandler5 To address this, brief interventions incorporating both behavioural and medical treatment in clinical settings such as the emergency department (ED), with a bridge to follow-up therapy, have been promoted for treating substance use disorders.
Patients with OUD are frequently seen in EDs for reasons such as opioid overdose, withdrawal, skin and soft tissue infections, endocarditis, osteomyelitis, and comorbid mental health issues. In many EDs, the primary option for managing OUD is passive referral to outpatient addiction clinics, where treatment with methadone or buprenorphine/naloxone would be started. A randomized controlled trial in the United States has suggested that patients with OUD are more likely to continue treatment if opioid agonist therapy is started in the ED than if they are only referred for treatment.Reference D'Onofrio, O'Connor and Pantalon6 Buprenorphine/naloxone is particularly well-suited for initiation in the ED setting because it is effective, easy to prescribe, and allows for safe home induction for patients who are not yet in withdrawal.Reference Lee, Grossman, DiRocco and Gourevitch7
There has been limited research on the initiation of buprenorphine/naloxone in the ED setting, with the major studies based in the United States. The goal of this study was to evaluate an ED-initiated buprenorphine/naloxone program with referral to an existing community-based addictions clinic, for patients in opioid withdrawal, in a Canadian context.
METHODS
This retrospective chart review study was approved by the institutional ethics review board of Lakeridge Health (Oshawa, ON). Lakeridge Health is a health system that encompasses four acute care community hospitals with EDs (Bowmanville, Oshawa, Port Perry, and Ajax/Pickering).
Program description
In this program, multiple training sessions for screening for opioid withdrawal and OUD and administering/prescribing buprenorphine/naloxone were given to ED health care providers. Patients in the ED between April 2017 and December 2017 were screened for OUD by health care providers if they presented to the ED with signs of opioid withdrawal or were opioid-seeking. To be eligible for the program, patients had to meet criteria for OUD using Diagnostic and Statistical Manual-5 (DSM-5) criteria and deemed as being in at least mild opiate withdrawal as assessed with the Clinical Opiate Withdrawal ScaleReference Wesson and Ling8 (COWS; a score greater than 5; refer to Appendix 1). Patients who had used opioids within the previous 12 hours or who were on methadone were not eligible for buprenorphine/naloxone treatment. At the time of this study, ED-specific protocols for the initiation of buprenorphine/naloxone were not available. Our protocol was developed and agreed by consensus among both ED and addictions health care providers (refer to Appendix 2 for the treatment algorithm). Patients who were eligible for and consented to treatment were given an initial dose of buprenorphine/naloxone in the ED (2–4 mg) and were witnessed by a nurse to ensure the medication was taken sublingually and had fully dissolved. Patients were re-assessed in 1–2 hours using the COWS score. If still in withdrawal, another 2–4 mg of buprenorphine/naloxone was administered sublingually based on the severity of withdrawal symptoms. The maximum dose administered in the ED was 8 mg. The patient was then discharged from the ED with a package containing information on opioid withdrawal symptoms, options for managing opioid withdrawal, contact information for clinics prescribing methadone and buprenorphine/naloxone, and information regarding outpatient case management programs. A buprenorphine/naloxone prescription with up to three daily observed doses, a request to the pharmacy to provide take-home naloxone, and referral were faxed to the rapid access addiction clinic (RAAC). Patients were advised to go to the RAAC the next day and make an appointment on the same day or next business day. The prescription was either filled at the pharmacy on-site with the RAAC or sent to another pharmacy of their choice.
Data collection
Charts of patients who were enrolled in the study were reviewed retrospectively (refer to Appendix 3 for the selection algorithm). Demographic information (age, sex, and housing status), information regarding substance use (previous rehabilitation for OUD, substance use other than opioids, concurrent psychiatric comorbidity, number of ED visits in the previous year, and percentage of ED visits related to substance use) were collected. We determined the number of referred patients who were seen in the RAAC post-ED discharge; average number of days from ED discharge to first RAAC appointment; ongoing RAAC follow-up and opioid agonist treatment at one, three, and six months post-index ED visit (confirmed with treatment providers); number of ED visits at one, three, and six months post-index ED visit (and percentage of visits related to substance use); and number of hospitalizations at one, three, and six months post-index ED visit (and percentage of hospitalizations related to substance use).
Statistical Analysis
All data were anonymized before analysis. Descriptive statistics, chi-square tests, and Kruskal-Wallis H tests were used to analyze the data. A p-value of less than 0.05 was used to determine significance. Statistical analyses were completed using SPSS (version 20).
RESULTS
The overall sample included 49 patients (mean age 37.0 years, standard deviation [SD] = 12.3). Fifty-seven percent of the study sample was male. Baseline patient characteristics for the overall sample are shown in Table 1. Of note, 24% of the overall sample had previous rehabilitation or supportive treatment for OUD, and 71% had at least one ED visit in the previous year (total of 119 visits among the sample, with 57% of the visits related to substance use). Approximately 45% had a concurrent psychiatric comorbidity, and 24% reported substance use other than opioids. Approximately 88% of these patients (n = 43) consented to receive buprenorphine/naloxone treatment in the ED and referral to an outpatient RAAC. Excluded patients included: patients who consented to only receive buprenorphine/naloxone in the ED for withdrawal management and not referral (6.1%); and patients who consented to referral only (6.1%).
Table 1. Overall patient characteristics
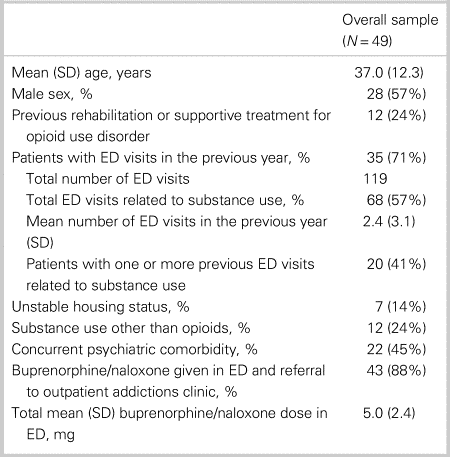
ED = emergency department; SD = standard deviation.
Data regarding outpatient RAAC follow-up and post-index ED visit health care utilization for patients in the program are shown in Table 2. Approximately 54% of patients referred for outpatient RAAC follow-up attended the initial appointment. The mean number of days from ED discharge until the first outpatient RAAC follow-up was 2.7 days (SD = 2.9). The mean age of this group was 37 years (SD = 12). At six months post-index ED visit, 35% had ongoing buprenorphine/naloxone treatment, 2.3% were weaned successfully off opioids, 16% started/stopped buprenorphine/naloxone treatment, and 47% did not show to an initial appointment.
Table 2. Outpatient addictions follow-up and health care utilization for patients given buprenorphine/naloxone and referral
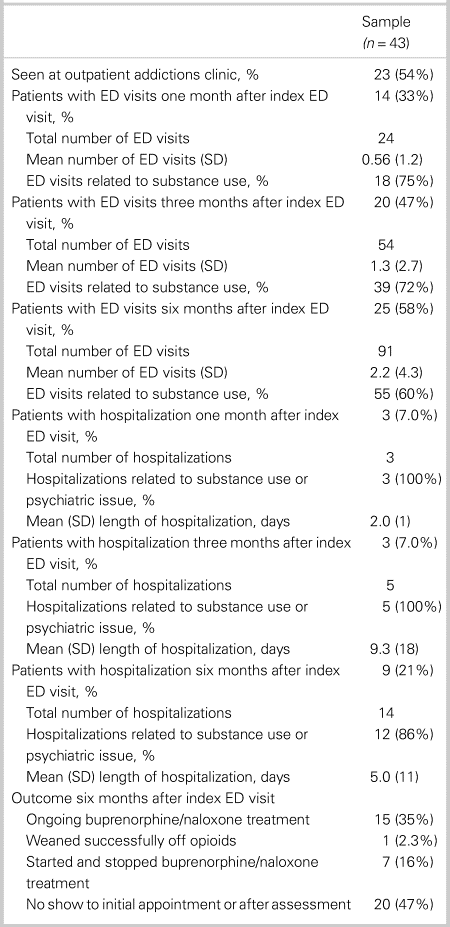
ED = emergency department; SD = standard deviation.
Table 3 shows the differences in health care utilization post-index ED visit between patients who continued with buprenorphine/naloxone treatment/weaned successfully off opioids compared with patients who started/stopped treatment or did not show up to the initial RAAC appointment. Between groups, there were no significant differences in terms of age (Kruskal-Wallis H test χ2(2) = 1.03, p = 0.60), sex (chi-square test χ2(2) = 4.77, p = 0.09), previous rehabilitation or supportive treatment for OUD (chi-square test χ2(2) = 1.49, p = 0.47), housing status (chi-square test χ2(2) = 0.11, p = 0.95), use of other substances than opioids (chi-square test χ2(2) = 1.01, p = 0.60), comorbid psychiatric conditions (chi-square test χ2(2) = 2.67, p = 0.26), and number of ED visits in the previous year (Kruskal-Wallis H test χ2(2) = 5.46, p = 0.07). A significantly lower proportion of patients in the ongoing treatment group had at least one ED visit at three months post-index ED visit (chi-square test χ2(2) = 8.17, p = 0.02). Patients in the ongoing treatment group had significantly fewer total ED visits at three months (Kruskal-Wallis H test χ2(2) = 9.98, p = 0.01), with a mean rank total number of ED visits of 15 for the treated group, 29 for the group that started/stopped treatment, and 25 for the group that did not show up to the RAAC appointment. Further, patients in ongoing treatment had significantly fewer total number of ED visits at six months (Kruskal-Wallis H test χ2(2) = 7.99, p = 0.02), with a mean rank total number of ED visits of 16 for the treated group, 31 for the group that started/stopped treatment, and 24 for the group that did not show up to the RAAC appointment. A significantly lower proportion of the ongoing treatment group had ED visits related to substance use at three months (chi-square test χ2(2) = 9.68, p = 0.01) and six months post-index ED visit (chi-square test χ2(2) = 8.43, p = 0.02). There were no significant differences between groups in terms of total number of ED visits at one month (Kruskal-Wallis H test χ2(2) = 5.16, p = 0.08), hospitalizations at one month (Kruskal-Wallis H test χ2(2) = 0.69, p = 0.71), hospitalizations at three months (Kruskal-Wallis H test χ2(2) = 0.74, p = 0.69), or hospitalizations at six months (Kruskal-Wallis H test χ2(2) = 2.78, p = 0.25).
Table 3. Health care utilization by outpatient RAAC outcome
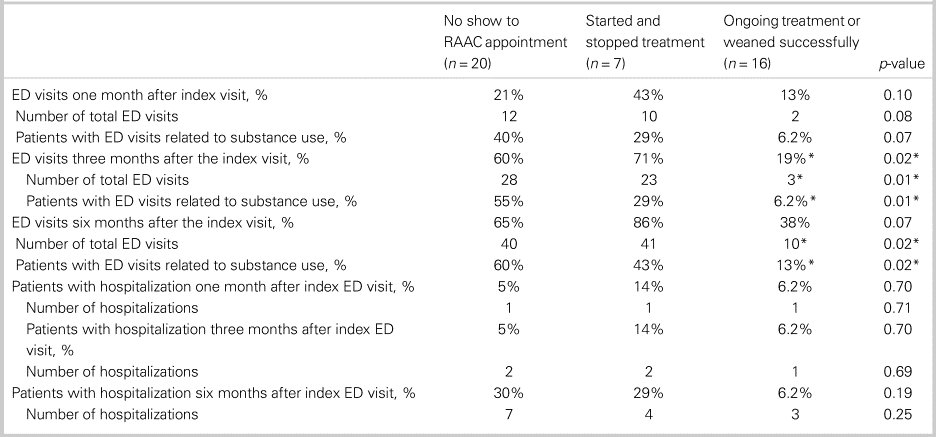
ED = emergency department; RAAC = rapid access addiction clinic.
*p < 0.05
DISCUSSION
To address the growing issue with OUD in the ED setting, we developed a program to screen for OUD among ED patients presenting in opioid withdrawal, offer buprenorphine/naloxone treatment, and facilitate a referral to an outpatient community-based RAAC. Our study showed that a majority of patients presenting with OUD in the ED setting (88%) were agreeable to starting buprenorphine/naloxone treatment and consented to referral for outpatient addictions follow-up, with 54% attending the initial appointment. Our results show that 35% of patients had ongoing buprenorphine/naloxone treatment and 2.3% were weaned successfully off opioids six months after the index ED visit. Our study showed significantly fewer ED visits at three and six months after the index visit among patients in ongoing treatment were successfully weaned off opioids compared with those who did not attend follow-up or started and stopped treatment.
There has been limited research examining outcomes from ED-initiated buprenorphine/naloxone programs with rapid referral to outpatient addiction clinics, with most of the research conducted in the United States. D'Onofrio and colleagues conducted a randomized controlled trial in the United States to test the efficacy of: 1) screening for opioid dependence and referral to treatment; 2) screening, brief intervention, and facilitated referral to community-based treatment services; and 3) screening, brief intervention, ED-initiated treatment with buprenorphine/naloxone, and referral to primary care for 10-week follow-up.Reference D'Onofrio, O'Connor and Pantalon6 Results showed that 78% of patients in the ED-initiated buprenorphine/naloxone group v. 37% in the referral group and 45% in the brief intervention group were engaged in treatment at one month that was a significant difference. In a follow-up analysis, D'Onofrio and colleagues showed that the ED-initiated buprenorphine/naloxone group continued to have a significantly higher proportion of patients engaged in treatment at two months, but there were no significant differences at 6 and 12 months.Reference D'Onofrio, Chawarski and O'Connor9 There were several key differences between our study and the study conducted in the United States: 1) we did not have a control group to assess treatment retention rates for patients who just received a referral; 2) the US study hired research associates to conduct screening for OUD in the ED with potential selection bias of patients recruited based on the time when the research associates were in the ED, and our study utilized existing resources (ED health care providers), making it more feasible in settings where funding is limited; 3) the US study was based at one urban teaching hospital ED, and our study was based at a network of four acute care community hospitals with EDs; 4) engagement in formal addiction treatment at 2, 6, and 12 months in the US study was based on self-report and not confirmed with treatment providers (prone to self-report bias), and our follow-up rates were confirmed with treatment providers; and 5) in the US study, after 10 weeks of office-based buprenorphine/naloxone treatment within the same centre, patients were transferred for ongoing maintenance treatment to either a community program/clinician or offered detoxification over two weeks. The US study showed no differences in groups after two months, which is when this transition of care occurred. This may also reflect the significant differences in the health care system between Canada and the United States. In Canada, residents are covered by public universal health insurance, with many health care services covered. Buprenorphine/naloxone is publicly funded in Canada for many patients: for example, in Ontario, coverage is provided for patients under 25 years of age, over 65 years of age, and those receiving social assistance, which greatly increases access for patients. In most Canadian provinces, there is no exemption required to prescribe buprenorphine/naloxone, which has helped reduce barriers to access, compared with the mandatory waiver in the United States.Reference Huhn and Dunn10 In the United States, most health care services are funded through private insurance, with a significant portion not having any coverage: in the US study, 22% did not have any health insurance that may have impacted treatment retention rates after 10 weeks when patients were transferred to other clinics and may not have had coverage or the ability to pay for buprenorphine/naloxone.
In addition, this study highlights a successful partnership between hospital EDs and existing buprenorphine/naloxone prescribers and pharmacies to provide rapid access to medication-assisted treatment. Given the scarcity of system funding, this approach may provide a cost-effective solution through utilization of existing community resources and is a model that is feasible in other communities. Expanding the use of ED-initiated buprenorphine/naloxone with rapid follow-up can help increase access to treatment options for patients with OUD, a chronic and relapsing condition that has significant mortality rates when untreated. Our study adds to the literature on “interim” opioid agonist treatment, in which buprenorphine/naloxone treatment is initiated while patients await more comprehensive addiction services. Given the increasing prevalence of OUD and mortality rates, it is important to initiate programs that reduce barriers to treatment access and reduce wait times for accessing treatment.
Limitations
Limitations of this study include a relatively small sample size within a single network of community hospitals that limited the precision and generalizability of these findings and a lack of a control group to compare what treatment retention rates would be without buprenorphine/naloxone initiation in the ED. Further research with a larger sample size, broad screening for OUD to ensure patients presenting with opioid-related complications are also included, and a longer follow-up are needed to evaluate patient outcomes fully. We were unable to follow-up with patients individually to determine reasons for not attending RAAC follow-up or for stopping buprenorphine/naloxone treatment, which would be an avenue for future research. Interventions that focus on assisting patients with navigating a complex health care system, arranging free transportation to the outpatient clinic, and addressing other social determinants of health should be considered in future research, as these factors may impact both initial follow-up and treatment retention rates.
CONCLUSIONS
In this study examining short-term follow-up after ED-initiated buprenorphine/naloxone with referral to an outpatient RAAC for patients presenting with opioid withdrawal, approximately 37% were in ongoing maintenance therapy or weaned off opioids. Patients with ongoing treatment had significantly fewer total ED visits at three and six months compared with patients who did not show up for follow-up or started/stopped treatment, which suggests that ED-initiated buprenorphine/naloxone is an effective intervention, but further research is needed.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2019.24
Competing interests
None declared.





