Background
An estimated 8 million low-risk chest pain patients present annually to emergency departments (EDs) in the United States.Reference Amsterdam, Kirk and Bluemke 1 The vast majority have nonlife-threatening problems. Current guidelines, nonetheless, recommend 6–8 hours of monitoring and serial troponin measurements with provocative testing performed within 72 hours in troponin-negative patients.Reference Amsterdam, Kirk and Bluemke 1 A cost-effective yet safe approach for early discharge of low-risk patients could significantly help reduce ED overcrowding and hospital admission rates without increasing cardiac morbidity and mortality.
Population Studied
The study prospectively enrolled 1,975 adults (≥18 years) who presented to two urban EDs with at least 5 minutes of chest pain consistent with acute coronary syndrome (ACS), where the attending physician planned to do serial cardiac troponin (cTn) testing. Patients with ST segment elevation myocardial infarction (also known as STEMI), with clear cause of chest pain other than ACS, or previously enrolled in the study, were excluded. Pregnancy, transfer from other hospitals, or cases where the attending physician considered recruitment inappropriate were among other exclusion criteria.
Study Design
ADAPT (2-hour ADP to assess patients with chest pain symptoms using contemporary troponins as the only biomarker) was a prospective observational study validating an ADP (which included a thrombolysis in myocardial infarction [TIMI] score, an electrocardiogram [ECG], and 0 and 2-hour cTnI as a single biomarker [Table 1]) used in the ASPECT (2-hour diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region) study.Reference Than, Cullen and Reid 2 Patients were considered low risk with a 1) TIMI score of zero, 2) no new ischemic changes on ECG, and 3) cTnI at 0 and 2 hours below the institutional cutoff level for an upper limit of normal. It was held in two urban hospitals in Brisbane, Australia, and Christchurch, New Zealand, between November 2007 and February 2011. Patients were followed for the occurrence of major adverse cardiac events (MACE) for up to 1 year. They also recorded data regarding further diagnostic testing or therapeutic interventions performed on this cohort for the 30 days following their presentations.
Table 1 Accelerated Diagnostic Protocol
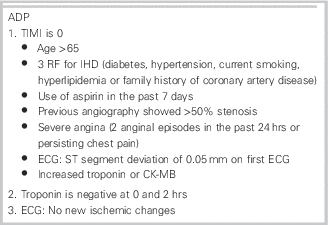
Outcomes
The primary outcome was a composite of MACE that occurred within 30 days after the first presentation. The adverse events included cardiovascular death, cardiac arrest, cardiogenic shock, emergency revascularization procedure, ventricular arrhythmia or atrioventricular block necessitating intervention, and acute myocardial infarction (MI).
Results
Follow-up was 100%. Of 1,975 patients, 302 (15.3%) had a MACE during the 30 days. The study identified 392 (20%) at low risk using the ADP. One of these patients (0.25%) had a MACE, that is, an MI diagnosed by an elevated troponin at 12 hours during the same ED visit. The sensitivity of the ADP was 99.7% (95% confidence interval [CI]: 98.1–99.9) with a specificity of 23.4% (95% CI: 21.4–25.5). The negative predictive value of the protocol was 99.7% (95% CI: 98.6–100), and the positive predictive value was 19% (95% CI: 17.2–21.0). This represents a negative likelihood ratio of 0.01 (0.002–0.1) (Table 2).
Table 2 Results
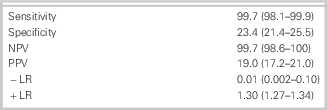
All three components of the ADP were needed to achieve sufficient sensitivity at an early time frame after presentation. There was a false-negative rate of 0.25% for all three components, including a TIMI score of 0 versus 3.2% when only troponins and ECG were used. In a secondary analysis, using a TIMI score of either 0 or 1 increased the percentage of patients categorized as low risk to 38.4% but resulted in nine false-negative results yielding a sensitivity of 97%, negative predictive value of 98.8%, specificity of 44.8%, and a positive predictive value of 24.1%.
Study Conclusion
The authors concluded that their ADP identifies patients with low-risk chest pain without an increase in MACE, and patients negative for the ADP are suitable for early discharge and short-term outpatient follow-up.
Commentary
Interest in the development of an ADP for low-risk chest pain in the ED has heightened recently with a number of publications touting various clinical decision instruments.Reference Christenson, Innes and McKnight 3 – Reference Than, Flaws and Sanders 6 The methodology and results of the ADAPT trial warrant similar interest. With the use of cTnI as the sole biomarker, the authors were able to achieve similar sensitivities as in the ASPECT trial (99.7% [95% CI: 98.1–99.9] versus 99.3% [95% CI: 97.9–99.8] ADAPT versus ASPECT, respectively), while improving on specificity (23.4% [95% CI: 21.4–25.5] versus 11.0% [95% CI: 10.0–12.2] ADAPT versus ASPECT).
There were limitations to this study. A significant weakness included the ADP study population that was a subset of the population studied in the earlier ASPECT trial. This was a homogeneous, primarily Caucasian group (90%), who had a relatively low rate of MACE of 15.3% at 30 days. In a Canadian study, the rate of MI and unstable angina at 30 days was 22%.Reference Christenson, Innes and McKnight 3 This higher disease prevalence would be expected to improve the positive predictive value of the ADP. High-sensitivity troponin assays (hs-cTnT) not used in this trial are increasingly becoming the standard. A recent study found similar sensitivities using this ADP with hs-cTnT but with improved specificity, up to 48.7% (95% CI: 46.1–51.3%).Reference Cullen, Mûller and Parsonage 7 Although increased sensitivity with the hs-cTnT assay might more likely have been expected, the increased specificity achieved here could perhaps be seen as unexpected and counterintuitive.Reference Jairam, Jones and Samaraie 8 , Reference Parsonage, Greenslade and Hammett 9 Of note, this study included both TIMI 0 and TIMI 1 patients as part of the ADP.
The authors demonstrated that by using their ADP, 20% of low-risk chest pain patients could theoretically be safely spared further workup through the ED with a sensitivity and negative predictive value of 99.7%. Nonetheless, 74.1% of the ADP-negative patients underwent further investigation, mostly stress tests, within 30 days. While some patients went on to have therapeutic and diagnostic procedures, outcome misclassification due to co-intervention may have been introduced here.Reference Hess and Jaffe 10
Questions arising from this study include how close to zero risk can one approach with inpatient assessment beyond negative troponins in the ED and whether further assessment is justified by the evidence, be it inpatient or outpatient, for the apparent low-risk patient, as suggested by this ADP. These may be especially relevant to jurisdictions, including Canadian ones, where access to timely outpatient cardiac follow-up may be lacking. In one study, 98.2% of TIMI 0 patients who underwent inpatient assessment subsequently demonstrated no coronary event at 30 days.Reference Hess, Agarwal and Chandra 11 Newman argued that in a population similar to this ADP negative group, the risk for subsequent 30-day MACE is in the order of 0.4%.Reference Newman 12 This risk was not further reduced by the performance of provocative testing, and, in fact, this testing often leads to percutaneous interventions, which, in the absence of positive biomarkers, may actually increase mortality.Reference Newman 13 Much of the practice was dictated by the American Heart Association 2010 guidelines, which reaffirmed the necessity for further confirmatory tests beyond ECGs and biomarkers, an opinion based predominantly upon expert consensus.Reference Greene 14 One might hope that a highly sensitive ADP, such as the one discussed, with improved specificity, and validated among a heterogeneous patient population, might further supplant the directive for these confirmatory tests in future guidelines.
Conclusion
The ADP validated in the ADAPT trial adds to the work on a clinical decision instrument for low-risk chest pain that might one day be developed. Physicians may eventually safely discharge a significant percentage of such patients after only a 2-hour assessment. Because the limited setting and narrow spectrum of patients from which this rule was derived provide only level III evidence as support,Reference Guyatt and Rennie 15 it will have to be validated with other patient groups and in different practice settings before it is universally adopted.
Competing interests: None declared.




