CLINICIAN'S CAPSULE
What is known about the topic?
Methemoglobinemia (MetHb) is an uncommon and potentially dangerous cause of cyanosis in the pediatric emergency department (ED), making its diagnosis a clinical challenge.
What did the study ask?
What are the clinical characteristics of patients with acquired MetHb in our pediatric ED?
What did this study find?
Pediatric ED MetHb presentation is rare but does exist. All our patients had common triggers or underlying conditions associated with MetHb.
What does this study matter to clinicians?
Awareness of key features associated with MetHb can help ED physicians diagnose and manage MetHb in cases of unusual pediatric cyanosis.
INTRODUCTION
Methemoglobinemia (MetHb) is an uncommon cause of cyanosis in the pediatric population that can cause significant morbidity and even death. MetHb occurs when the iron atom in hemoglobin loses one electron to an oxidant, and the Fe II, or oxidized state, of iron is transformed into Fe III. Because Fe III iron is unable to bind oxygen, and the oxygen affinity of any remaining Fe II in the hemoglobin is increased, a left shift occurs in the oxygen dissociation curve, resulting in functional anemia and impaired oxygen delivery to the tissues.Reference Price, Nelson, Lewin, Howland, Hoffman, Goldfrank and Flomenbaum1 Most cases of MetHb are acquired, induced by agents that cause an increase in the production of methemoglobin, making it a potentially predictable and preventable condition.Reference Price, Nelson, Lewin, Howland, Hoffman, Goldfrank and Flomenbaum1
MetHb is a rare but potentially dangerous cause of cyanosis in pediatric emergency department (ED) patients. It is important for clinicians to be aware of the key features of patient history and clinical presentation that should raise suspicion for the diagnosis of MetHb. The aim of our study was to improve the clinician's ability to recognize the potential for MetHb in pediatric ED patients and to avoid overlooking this important cause of cyanosis.
METHODS
Study design and subjects
We conducted a case series using a health records review. Records were obtained from pediatric patients with acute acquired MetHb in the ED of the Hospital for Sick Children in Toronto, Canada, from January 1, 2007, to April 30, 2018. This hospital is a large, urban, tertiary pediatric center with approximately 75,000 ED visits per year. Patients were identified by searching for the diagnosis of ”methemoglobinemia” in the electronic health record system as well as from laboratory results of methemoglobin levels ordered from the ED. Cases were included only if their presentation and diagnosis were made in our ED and methemoglobin saturation ≥5%. While there is no consensus on the lower limit of methemoglobin that should be considered MetHb, ≥5% reflects the consensus cutoff at our center.
Variables
The following variables were extracted from the electronic health record: date of ED visit, age, gender, medical history, physical findings, vital signs, laboratory results, treatment plan, disposition, and prognosis.
Ethics
The study was approved by the Research Ethics Board of the hospital.
RESULTS
Ten patients were diagnosed with acquired MetHb during the study period. Median age was 5.5 years (IQR = 2.3, 9.5) with seven males and three females. The clinical presentation and management of our patients is presented in Table 1.
Table 1. Clinical presentation and management of patients with acquired methemoglobinemia
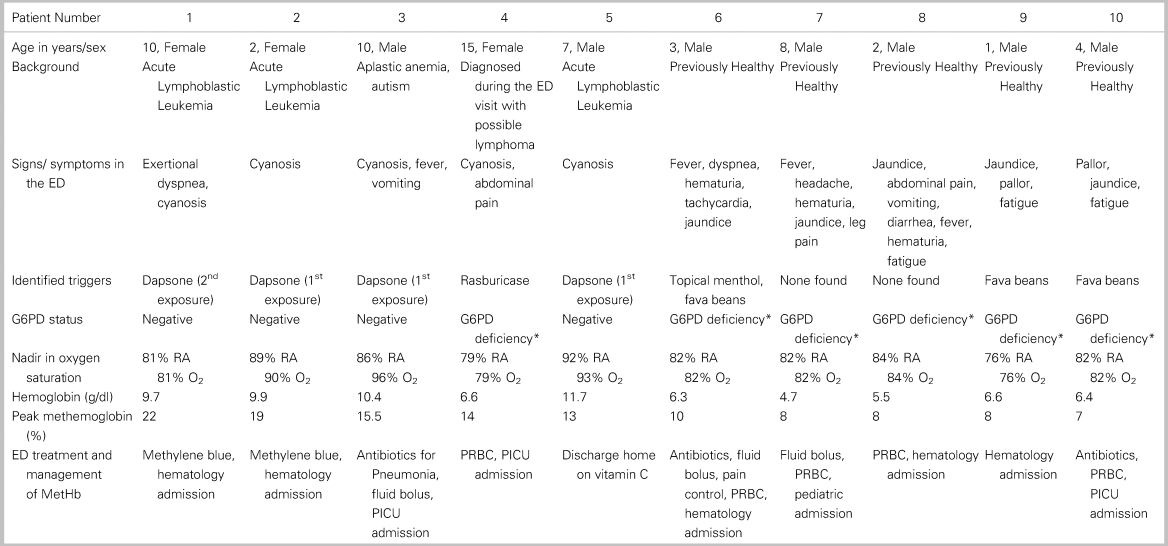
ED = emergency department; G6PD = glucose-6-phosphate dehydrogenase; RA = room air; O2 = saturation with supplemental oxygen; PICU = pediatric intensive care unit; PRBC = packed red blood cells; *G6PD = deficiency not previously known, diagnosed in the ED.
Four patients (patients 1, 2, 3, 5) had a known background of underlying disease: three with acute lymphoblastic leukemia, one aplastic anemia, and one newly diagnosed lymphoma. In each of these patients, there was an obvious pharmacological trigger for the MetHb (dapsone). Patient 4 was diagnosed with suspected lymphoma in the ED and given rasburicase in the ED to prevent tumor lysis syndrome; she subsequently developed MetHb. Patient 4 was also found to have a previously unidentified glucose-6-phosphate dehydrogenase (G6PD) deficiency. The remaining five patients (patients 6–10) were previously healthy and presented to our ED with a clinical picture of acute hemolytic anemia including a variable combination of cyanosis, fatigue, jaundice, fever, and dyspnea. They were all found to have previously undiagnosed G6PD deficiency. A probable trigger was found for three of these patients: two were exposed to fava beans, and one was exposed to both topical menthol and fava beans.
Notably, all patients, except patient 3 who also was diagnosed with pneumonia, had a negligible improvement if any in oxygen saturation in response to supplemental oxygen.
Treatment consisted of packed red blood cell transfusion for five of the patients. Methylene blue was given to two patients with methemoglobin saturation of 22% and 19%. One patient received vitamin C upon discharge, and one received supportive care only. In all of our patients, methemoglobin saturation percentages normalized after diagnosis and treatment, and all patients survived the episode of acute acquired MetHb to eventual discharge home.
DISCUSSION
The cases in our series are consistent with previous studies of MetHb, in particular, the clinical features on presentation, the increased risk due to specific triggers, and the persistence of hypoxia despite supplemental oxygen.
Previous pediatric studies reported diverse potential contributors to this condition including: underlying conditions such as G6PD, age <36 months, diarrhea, malnutrition and sepsis, exposure to certain foods and medications such as dapsone (antibiotic), local anaesthetics, rasburicase (recombinant urate oxidase), aniline dyes, and high levels of nitrate in water supplies.Reference Price, Nelson, Lewin, Howland, Hoffman, Goldfrank and Flomenbaum1–Reference Guay4
The presenting symptoms of acute acquired MetHb depend on methemoglobin saturation percentage.Reference Cortazzo and Lichtman5 As methemoglobin saturation levels rise, mild symptoms, such as headache, dyspnea, fatigue, dizziness, and anxiety develop. At saturations >40% coma, respiratory depression, convulsions, arrhythmias, and death may occur.Reference Bucklin and Groth6 The diagnosis is suggested in patients with sudden onset of cyanosis and hypoxia that fails to improve with an increased fraction of inspired oxygen.Reference Barker, Tremper and Hyatt7 A dark red, chocolate brown, or blue color of blood observed during phlebotomy and clinical cyanosis in the presence of a normal calculated arterial pO2 would also be suggestive of MetHb.
Exploring a history of potential MetHb trigger exposures (pharmaceutical agents, precipitating foods) is essential in the early identification of patients presenting with cyanosis. The pharmacologic triggers (rasburicase, dapsone, and menthol) identified in our case series are well-known MetHb triggers. Rasburicase and dapsone are commonly used in the management of pediatric oncology patients but may be less familiar to many emergency physicians. It is important that emergency physicians are aware that pediatric patients with hematologic malignancies may be at increased risk for MetHb due to their treatment medications.
As demonstrated in our case series, pulse oximetry can be misleading and inaccurate in cases of acute MetHb and should not be used to make the diagnosis.Reference Barker, Tremper and Hyatt7 High concentrations of methemoglobin may cause the pulse oximeter to display approximately 85% regardless of the true hemoglobin oxygen saturation due to its inability to read true methemoglobin saturation percentage.Reference Barker, Tremper and Hyatt7
The management of acute, acquired MetHb is variable and depends on clinical symptoms, methemoglobin and hemoglobin saturation percentage, and underlying conditions such as G6PD deficiency. The first step is to suspend use of the offending agent(s) if identified. In an asymptomatic patient, with methemoglobin levels <20%, no further action is usually needed; normally metabolizing red blood cells will reduce the methemoglobin within several hours. When intervention is needed, the most widely accepted treatment of MetHb is administration of 1–2 mg/kg methylene blue intravenously over 5 minutes. Methylene blue causes enzymatic reduction of methemoglobin by means of nicotinamide adenine dinucleotide phosphate (NADPH)-methemoglobin reductase. The response is usually rapid, and the dose may be repeated in 1 hour if high saturation percentage of methemoglobin persist. Methylene blue administration in patients with known G6PD deficiency is controversial, due to the risk of hemolytic anemia.Reference Price, Nelson, Lewin, Howland, Hoffman, Goldfrank and Flomenbaum1 Recommendations vary between administering methylene blue in a normal dose,Reference Price, Nelson, Lewin, Howland, Hoffman, Goldfrank and Flomenbaum1 administering it at a low dose combined with ascorbic acid,Reference Price, Nelson, Lewin, Howland, Hoffman, Goldfrank and Flomenbaum1 or not using it at all and providing ascorbic acid only,Reference Rosen, Johnson, McGehee and Beutler8 Severe MetHb may benefit from exchange transfusion and/or hyperbaric oxygen.Reference Patnaik, Natarajan, James and Ebenezer9 Patients with severe MetHb should be managed in the intensive care unit.
CONCLUSION
Diagnosis and management of acute, acquired MetHb in the ED requires a high level of suspicion, and a background knowledge of the common precipitants and underlying conditions associated with this condition. Our study highlights the fact that MetHb should be considered in pediatric patients presenting with cyanosis and persistent hypoxia. Exposure to known precipitants (e.g., medications and foods), particularly in the setting of active treatment for malignancy or with symptoms of hemolytic anemia should further increase suspicion. We hope this case series will help ED physicians to recognize MetHb as a potential reversible cause of cyanosis in pediatric patients.
Competing interests
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.



