Introduction
Sweat bees (Halictidae) are arguably the most socially diverse of all animal taxa, their social behaviour ranging from more or less egalitarian communal societies to hierarchical eusocial societies based on the reproductive division of labour between queens and workers (Schwarz et al. Reference Schwarz, Richards and Danforth2007). Eusociality is by far the best-studied form of reproductive behaviour in sweat bees (Schwarz et al. Reference Schwarz, Richards and Danforth2007). In a typical, annual, eusocial colony cycle, queens raise a first brood of mostly females that, upon reaching adulthood, remain in their natal nest as workers and help their mother to raise a second, reproductive brood that includes future queens. A key aspect of sweat bee eusociality is the production of at least two broods per year: a first brood comprising workers and a few males, and a subsequent brood of additional males and future queens. The worker brood may be small, containing as few as a single worker (Packer Reference Packer1990) or there may be successive worker broods and hundreds of individual workers (Packer et al. Reference Packer, Coelho, Mateus and Zucchi2003), but a eusocial colony cycle always requires at least two phases of brood production and is therefore bivoltine or multivoltine (Schwarz et al. Reference Schwarz, Richards and Danforth2007). In contrast, solitary species can be univoltine, bivoltine, or multivoltine as successive generations of females raise their offspring.
Phylogenetic analyses of halictid bees suggest that solitary breeding is ancestral for the group and that there have been at least two origins of eusociality and multiple reversions to solitary behaviour (Brady et al. Reference Brady, Sipes, Pearson and Danforth2006; Gibbs et al. Reference Gibbs, Brady, Kanda and Danforth2012; Lepeco and Goncalves Reference Lepeco and Goncalves2022). Transitions to eusociality seem to be favoured during periods of global warming or range expansions into warmer climates, when daughters emerge early enough in the breeding season to raise their own offspring before the end of summer foraging seasons (Brady et al. Reference Brady, Sipes, Pearson and Danforth2006). Figure 1 illustrates a hypothetical scenario for evolutionary transitions from solitary to eusocial behaviour in sweat bees, as well as scenarios for reversals from eusocial to solitary breeding. Transitions to eusociality likely involved a two-step process, the first step being a change from univoltine to bivoltine solitary behaviour and the second being a change from solitary to eusocial behaviour (Brady et al. Reference Brady, Sipes, Pearson and Danforth2006). In univoltine sweat bees, daughters are thought to enter diapause shortly after eclosing as adults, preparatory to becoming foundresses and raising their own brood the following year (Sakagami et al. Reference Sakagami, Maeta, Nagamori and Saito1993). A switch from univoltinism to bivoltinism therefore requires that newly emerged adult daughters forego diapause and begin raising their own offspring, the second brood of the year (Hunt et al. Reference Hunt, Kensinger, Kossuth, Henshaw, Norberg, Wolschin and Amdam2007). In the second step, the change from bivoltine solitary to bivoltine eusocial behaviour supposes that, instead of laying their own eggs, brood 1 daughters redirect their brood care behaviours towards offspring that develop from eggs laid by their mother. The origins of eusocial behaviour in sweat bees therefore require a preadaptive step involving a change from univoltine to bivoltine or multivoltine phenology.
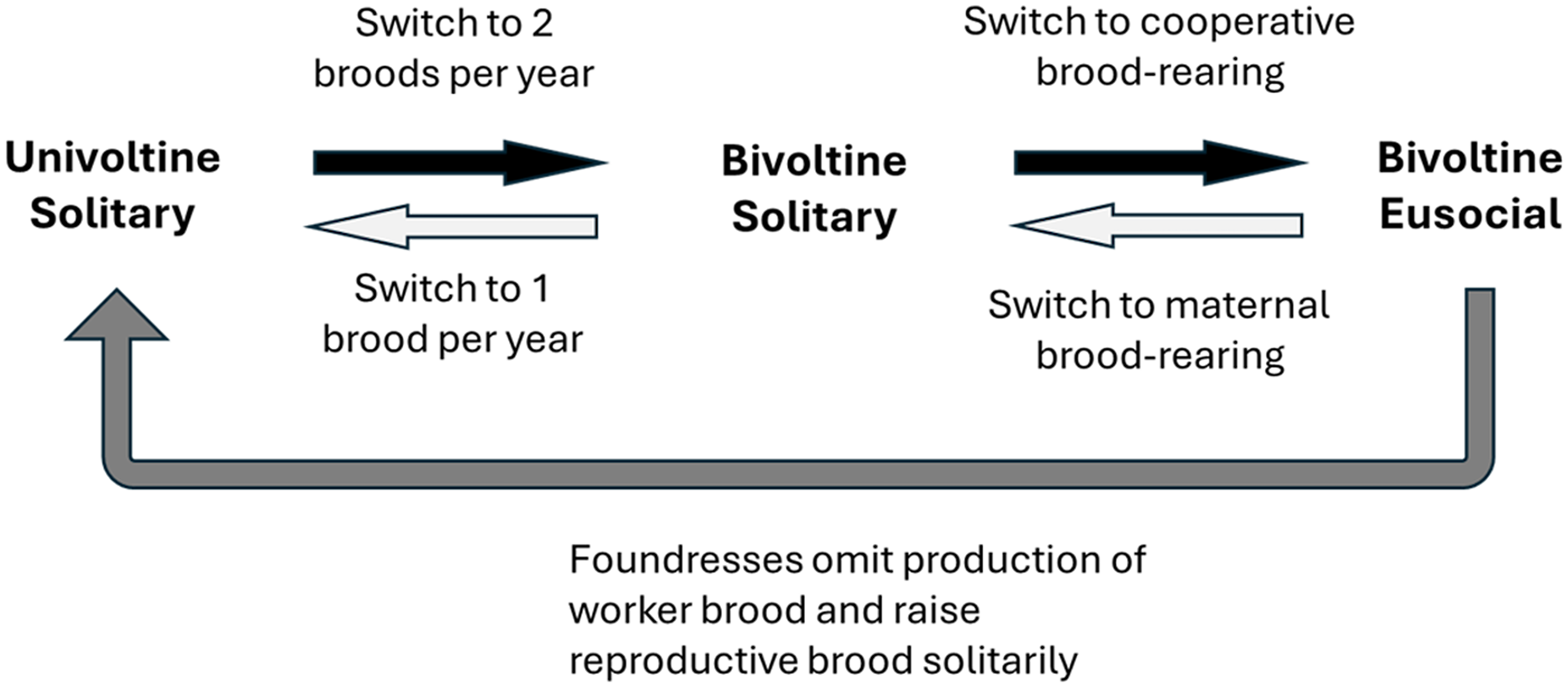
Figure 1. Hypothetical behavioural changes in evolutionary transitions between solitary and eusocial behaviour in sweat bees. Univoltine solitary bees produce one brood each year, and all brood care is maternal. Bivoltine solitary bees produce two broods per year, and all brood care is maternal. Bivoltine eusocial bees produce two broods per year, and brood 2 is raised cooperatively. Evolutionary transitions from solitary to eusocial behaviour require two major steps. Reversals from eusociality to bivoltine solitary behaviour require only one step. Reversals from eusociality to univoltine solitary behaviour might involve two steps, recapitulating in reverse, the original transition to eusociality. Alternatively, reversals to univoltine solitary behaviour might occur in a single step when nest foundresses omit production of a worker brood and instead raise reproductive brood.
Reversals to solitary behaviour in sweat bees seem to be favoured when breeding seasons become shorter, for instance, when populations expand into higher altitudes or latitudes (Sakagami and Munakata Reference Sakagami and Munakata1972; Eickwort et al. Reference Eickwort, Eickwort, Gordon, Eickwort and Wcislo1996). Figure 1 suggests two scenarios for evolutionary reversals. First, evolutionary reversals could recapitulate in reverse the two steps hypothesised in the origins of eusociality, with a switch from bivoltine eusociality to bivoltine solitary behaviour, followed by a switch to univoltine solitary behaviour. Alternatively, both steps might occur simultaneously if foundresses omit the production of a worker brood and simply raise a brood of reproductive daughters and sons, much as seems to occur in high-altitude populations of facultatively solitary sweat bees (Sakagami and Munakata Reference Sakagami and Munakata1972; Eickwort et al. Reference Eickwort, Eickwort, Gordon, Eickwort and Wcislo1996). Reversals from bivoltine eusociality to bivoltine solitary behaviour do not require evolutionary changes in phenology, but reversals to univoltine solitary behaviour do require such a change. Testing hypotheses about the sequence of behavioural changes involved in evolutionary transitions between solitary and eusocial behaviour therefore requires detailed information about both phenology and reproductive behaviour.
A major impediment to behavioural studies of sweat bee nesting behaviour is that the nests of most species in most places are difficult to find. Systematic trapping studies over the entire course of a flight season can provide a partial solution to this problem. Although nest-based studies remain the “gold standard” for studies of colony social organisation because they allow direct observations of female behaviour, specimens acquired in trapping studies can yield reliable information about foraging flight phenology (univoltine, bivoltine, or multivoltine patterns) and female traits related to reproductive behaviour and colony social organisation (Packer et al. Reference Packer, Gravel and Lebuhn2007). Moreover, direct comparisons of nest-based versus trap-based data demonstrate that the latter yield highly consistent, albeit less detailed, information about female reproductive behaviour (Richards et al. Reference Richards, Vickruck and Rehan2010, Reference Richards, Onuferko and Rehan2015; Corbin et al. Reference Corbin, Awde and Richards2021). Studies based entirely or in part on trapping data have allowed both solitary and eusocial behaviour to be better characterised in multiple species and in populations that would otherwise have remained behaviourally unstudied – for example, Halictus ligatus and H. poeyi (Dunn et al. Reference Dunn, Mitchell and Packer1998), H. tripartitus (Packer et al. Reference Packer, Gravel and Lebuhn2007), and L. hitchensi and L. ellisiae (Corbin et al. Reference Corbin, Awde and Richards2021).
Trapping studies are particularly useful for distinguishing between solitary and eusocial behaviour. Pan traps, in particular, have the advantage of being highly attractive to foraging female sweat bees (Rutgers-Kelly and Richards Reference Rutgers-Kelly and Richards2013), and the abundance of foragers in pan traps reflects the level of foraging activity observed at nests (Richards et al. Reference Richards, Vickruck and Rehan2010, Reference Richards, Onuferko and Rehan2015; Corbin et al. Reference Corbin, Awde and Richards2021). This means that trapped specimens can provide reliable information for evaluating whether a population is univoltine, bivoltine, or multivoltine. Eusocial sweat bees are never univoltine, so univoltine populations can be presumed to be solitary. Distinguishing between bivoltine solitary and bivoltine eusocial behaviour rests on reproductive trait comparisons of females collected during the two brood provisioning phases. We refer to the first and second phases of brood provisioning activity in bivoltine populations as P1 and P2 (Fig. 2). In a bivoltine solitary population, P1 females represent nest foundresses foraging to provision brood 1, whereas P2 females mostly represent brood 1 daughters foraging to provision brood 2 (Fig. 2). Because both P1 and P2 females raise their own brood, they are predicted to be similar in size, wear, and ovary development. In a bivoltine eusocial population, most P1 females would be foundress queens, whereas most P2 females should be workers. Therefore, P1 females should be larger, more worn, and have higher ovary development than P2 females do. In this way, by examining the abundance and female traits of trapped bees, we can infer both flight phenology and whether females breed solitarily or eusocially.
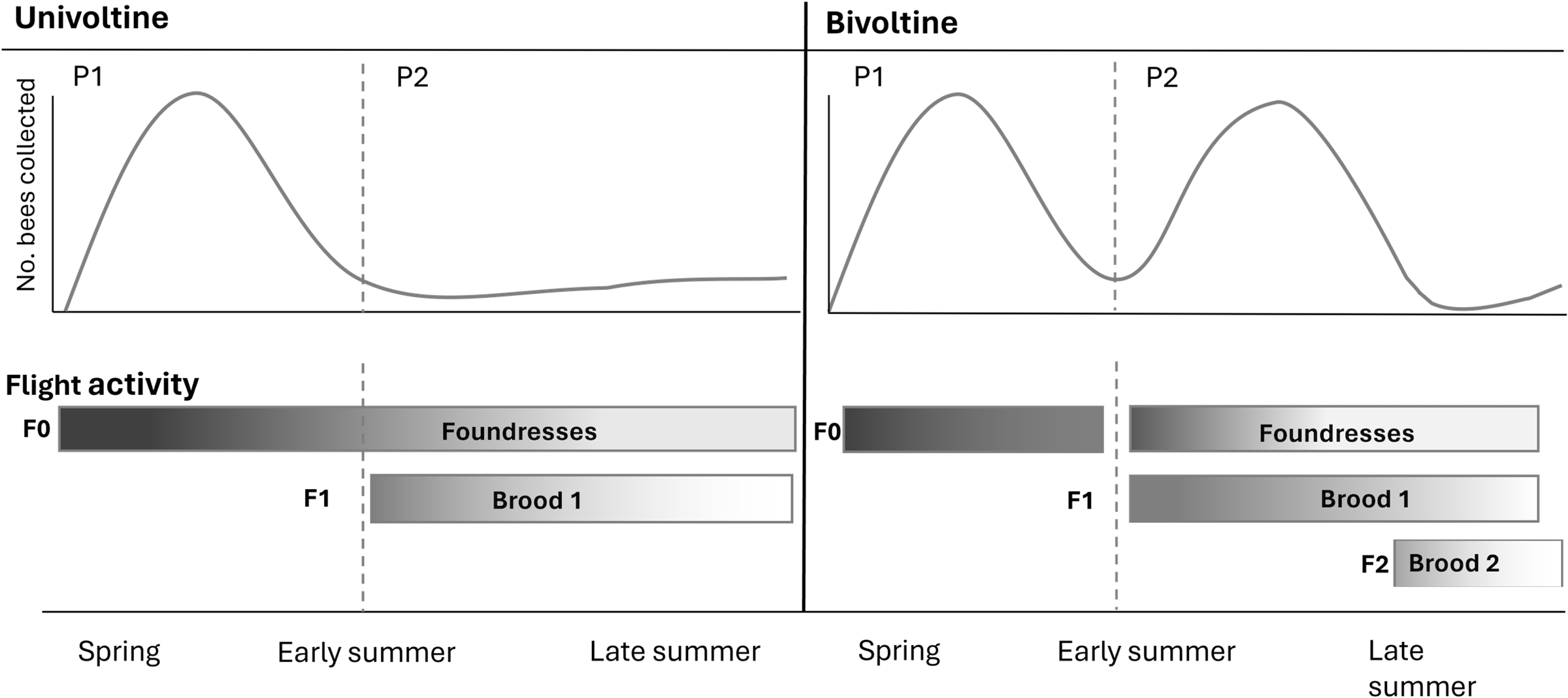
Figure 2. Phenology graphs and timelines illustrate univoltine and bivoltine activity patterns in sweat bees. Top graphs: Predicted abundances of females collected in traps from spring through late summer. Bottom timelines: Timelines depict foraging activity by foundresses and offspring, with darker shading indicating higher bee abundance. Phase 1 begins in spring when overwintered foundresses (F0) begin provisioning brood 1. In univoltine sweat bees, foundresses forage and provision brood until moribund, generating a single prominent peak in activity during phase 1 and an extended tail of long-lived foundresses that survive into phase 2. Brood 1 females do not provision brood. In bivoltine sweat bees, there is a hiatus in flight activity between phases 1 and 2, when foundresses temporarily halt provisioning activity after completing brood 1 before resuming provisioning of brood 2. In phase 2, females of brood 1 (F1) provision brood 2. In both univoltine and bivoltine populations, bee abundance in traps may increase towards the end of the summer, when late-emerging daughters emerge to feed before diapause.
A particularly interesting clade for studying transitions between solitary and eusocial behaviour is the subgenus Lasioglossum (Leuchalictus) because it includes a mix of species with univoltine and bivoltine phenologies, as well as solitary, communal, and eusocial behaviour (Table 1). In the present study, we focus on two Holarctic L. (Leuchalictus) species, L. leucozonium (Schrank) and L. zonulum (Smith), that are generally considered to be univoltine and solitary across their European and North American ranges (McGinley Reference McGinley1986; Pesenko et al. Reference Pesenko, Banaszak, Radchenko and Cierzniak2000). Neither species appears to have been the subject of detailed behavioural studies, although Pesenko et al. (Reference Pesenko, Banaszak, Radchenko and Cierzniak2000) summarised known information. In Poland, the univoltine flight phenology of L. leucozonium begins when females establish nests and begin brood provisioning in May (Pesenko et al. Reference Pesenko, Banaszak, Radchenko and Cierzniak2000). Peak brood provisioning occurs in late June, after which female abundance declines steadily until early August. The first adult males appear in late June, with male abundance peaking in late August. Female abundance also increases again in late August, and this likely represents the time when adult brood leave their natal nests to mate and feed before overwintering. The nesting behaviour of L. leucozonium has not been studied in detail, although Pesenko et al. (Reference Pesenko, Banaszak, Radchenko and Cierzniak2000) commented that nests contain 6–15 brood cells. Zayed et al. (Reference Zayed, Constantin and Packer2007) excavated 32 nests in southern Ontario, Canada, noting that they contained an average of 8.35 brood and were produced solitarily. Genetic analyses suggest that North American populations of L. leucozonium descend from very few propagules, possibly only a single female, carried in soil ballast of ships from western Europe sometime in the eighteenth or nineteenth centuries (Zayed et al. Reference Zayed, Constantin and Packer2007); it would be expected that this species is univoltine and solitary in North America.
Table 1. Phenology and reproductive behaviour summarised for Lasioglossum (Leuchalictus). Type of study refers to observations focused on females at their nests or females collected on flowers or while foraging

* As L. esoense.
† Bivoltinism of L. majus in Poland is suggested by the flight phenology presented in Pesenko et al. (Reference Pesenko, Banaszak, Radchenko and Cierzniak2000), fig. 324.
Pesenko et al. (Reference Pesenko, Banaszak, Radchenko and Cierzniak2000) also summarised the univoltine phenology of L. zonulum. In Poland, females establish nests and begin brood provisioning in early May, with peak brood provisioning occurring in late June and July, followed by a decline in female abundance. The first males appear in mid-June, and their numbers increase to a peak in August and early September. A second peak in female abundance occurs in August and early September and likely represents feeding and mating activity of newly eclosed adults before overwintering. Golubnichaya and Moskalenko (Reference Golubnichaya and Moskalenko1992) observed female behaviour at a nesting aggregation of 34 nests in Ukraine, one of which was excavated. Females nested solitarily, raising one brood per year, and daughters remained in the maternal nest to overwinter. The following spring, most daughters dispersed to find their own nests, but sometimes they refurbished their natal nest.
In this study, we used trapped specimens of L. leucozonium and L. zonulum to characterise phenology and solitary reproductive behaviour of several populations in Canada. Specimens of both species were collected as part of a long-term monitoring study in southern Ontario (Richards et al. Reference Richards, Rutgers-Kelly, Gibbs, Vickruck, Rehan and Sheffield2011; Onuferko et al. Reference Onuferko, Skandalis, León Cordero and Richards2018), and specimens of L. zonulum were collected as part of a shorter monitoring study in southern Alberta (Vickruck et al. Reference Vickruck, Best, Gavin, Devries and Galpern2019). Given that some members of Lasioglossum (Leuchalictus) exhibit both solitary and eusocial behaviour (Table 1), we first confirmed the solitary status of all three populations. We found that L. leucozonium exhibits univoltine solitary behaviour in southern Ontario, whereas L. zonulum exhibits bivoltine solitary behaviour in southern Ontario and univoltine solitary behaviour in southern Alberta. We then compared traits of females collected in P1 and P2 to examine similarities and differences between univoltine and bivoltine solitary behaviour.
Methods
Collection methods
In southern Ontario, bees were collected as part of a long-term monitoring study initiated in 2003 (Rutgers-Kelly and Richards Reference Rutgers-Kelly and Richards2013) that focused on several naturalisation sites in St. Catharines (GPS coordinates 43.123, –79.237, 2–9 trapping transects per year), Port Colborne (42.923, –79.258; 1–3 transects per year), and Wainfleet (42.884, –79.376; 1 transect per year). All three sites are former landfills restored as grassy meadows, with mostly open vegetation and flowers blooming throughout the bee flight season, supporting diverse bee communities (Onuferko et al. Reference Onuferko, Kutby and Richards2015). Analyses of phenology were limited to pan-trap samples, but specimens collected by sweep netting and aerial netting were used to augment sample sizes for the evaluation of female reproductive traits (Table 2); details of all three collecting methods are provided in Richards et al. (Reference Richards, Rutgers-Kelly, Gibbs, Vickruck, Rehan and Sheffield2011). To briefly summarise, pan traps (170 g Solo brand, PS6-0099 plastic bowls; Solo, Lake Forest, Illinois, United States of America) were filled with water and a small amount of detergent (5 drops of Blue Dawn dish soap (Procter & Gamble, Cincinnati, Ohio, United States of America) per litre of water). At any particular sampling location, 30 traps were placed at 3-m intervals along space-filling or linear transects, alternating bowl colours (blue, yellow, and white). Traps were laid out by 09:00 hours local time, and the contents were collected after 15:00 hours (after 16:00 hours local time in later years) on days without rainfall. Collections occurred weekly or biweekly, from mid-April or early May to September, over 14 years between 2003 and 2018 (excluding 2007 and 2014). Occasionally, sweep or aerial netting provided additional female specimens in the same collection weeks as pan-trapping weeks; these specimens were used only for the examination of female traits. Collection weeks were numbered using the WEEKNUM function in Microsoft Excel, with week 1 occurring in mid-April. All bees were stored in 70% ethanol and then pinned and identified using the key from McGinley (Reference McGinley1986). The term “collection” refers to the set of bees collected at a particular trapping site on a particular day. In total, bees were trapped in 954 collections in southern Ontario, but not all transect locations were collected in all weeks or years.
Table 2. Collection information for all three populations of sweat bees, Lasioglossum (Leuchalictus), examined in this study. No Ontario collections occurred in 2007 or 2014. Note that for the Ontario samples, week precisely describes the timing of a one-day collection, whereas for the Calgary samples, week refers to the midpoint of an extended collection period, usually lasting one week.

* 169 females caught in pan traps, 28 females caught with nets.
† 267 females caught in pan traps, 9 females caught with nets.
In southern Alberta, specimens were collected in 2016 as part of a separate project (Vickruck et al. Reference Vickruck, Best, Gavin, Devries and Galpern2019) and were kindly provided by Dr. Paul Galpern, University of Calgary (Calgary, Alberta, Canada). Collections were carried out near Calgary (GPS 50.663, –113.483); the agriculturally intensive landscape in this area comprises crop fields and wetlands. Collections began after crop seeding had been completed in June and ended before crop swathing in August. Both pan traps (12 oz., New Horizons, Waldorf, Maryland, United States of America) and blue vane traps (SpringStar LLC, Woodinville, Washington, United States of America) were used. Traps were filled with propylene glycol, and specimens were collected from the traps after 5 and 14 days of deployment (average 7.7 ± 2.5 days). For Alberta specimens, the term “collection” refers to all the bees collected in a single set of traps at a single location over a 5- to 14-day interval, so the week numbers assigned to Alberta specimens are less precise than those assigned to specimens from Ontario. In total, bees were trapped in 677 collections in Alberta. Specimens collected in Alberta were stored in 70% ethanol until pinned and identified by Dr. Lincoln Best, using keys listed in Vickruck et al. (Reference Vickruck, Best, Gavin, Devries and Galpern2019), shown in supplementary tables. Vouchers were deposited in the Invertebrate Section of the Museum of Zoology, Department of Biological Sciences, University of Calgary.
Phenology
Figure 2 compares univoltine and bivoltine phenologies of solitary sweat bees, comparing terminology used here and in previous literature to describe bee nesting cycles. In order to compare univoltine and bivoltine life histories, we divided the nesting cycle into two phases, based on the earliest appearance of males in trap collections. (Lasioglossum males do not overwinter, so their first appearance is a reliable indication that the first brood of the year is reaching adulthood.) We refer to the period before first male emergence as P1 and to the period following first male emergence as P2. In both univoltine and bivoltine sweat bees, P1 foragers are overwintered nest foundresses (also referred to as the F0 generation; Fig. 2). P2 foragers comprise a mix of old foundresses that are still foraging and male and female offspring (F1 generation) that leave the natal nest to feed themselves before overwintering, leading to a short increase in bee abundance during P2. Towards the end of the summer, newly eclosed adults of brood 2 may leave their nests to feed themselves before overwintering.
To infer the phenology of each population, we plotted average numbers of bees per collection per week (only those collected via pan traps in Ontario and via pan and blue vane traps in Alberta; Onuferko et al. Reference Onuferko, Skandalis, León Cordero and Richards2018). Specimens collected in 2003–2018 in Ontario were pooled over the years because L. leucozonium and L. zonulum are low-abundance species at our sites, with relatively few specimens captured each year. In contrast, for specimens from Alberta, where L. zonulum is highly abundant, we focused on specimens collected in a single year, 2016. The less precise week numbers of Alberta specimens mean that bees from the earliest collections are recorded as being from week 9 but could have been trapped up to a week earlier, so week 9 does not precisely delineate the beginning of spring foraging activity.
Female reproductive traits and colony social organisation
We measured female traits related to reproductive behaviour and colony social organisation (body size, wear, and ovary development), following methods developed for pinned specimens (Packer et al. Reference Packer, Gravel and Lebuhn2007; Richards et al. Reference Richards, Vickruck and Rehan2010; Corbin et al. Reference Corbin, Awde and Richards2021). All female specimens collected from Ontario were dissected. For L. zonulum specimens from Alberta, a set of 157 specimens was randomly selected for measurement and dissection using a random number generator before P1 and P2 dates were inferred.
Pinned specimens were examined using a stereomicroscope (Zeiss Stemi SV11, Zeiss, Oberkochen, Germany) equipped with an ocular micrometre, at 6–66× magnification, with separate corrections depending on the magnification used. Female size was measured as head width (distance across the widest part of the head, including eyes) and length of the forewing costal vein. Because these two measures were strongly correlated, we present results based on head widths. Degree of mandibular wear (MW) is expected to reflect the extent of activities such as digging brood cells and was assessed on a scale from 0 (mandible and apical tooth pointed and in pristine condition) to 5 (mandible completely blunt with no apical tooth). Wing wear (WW) is expected to reflect the extent of flight activity because bees accumulate wing damage over time (Cartar Reference Cartar1992; Foster and Cartar Reference Foster and Cartar2011) and was assessed on a scale from 0 (pristine wing margin along distal edge) to 5 (distal edge destroyed). Total wear (TW) was calculated by summing MW and WW for a combined score from 0 to 10. Females with a total wear score of 1 or less (TW ≤ 1) were categorised as unworn, whereas females with a total wear score of at least 2 (TW ≥ 2) were categorised as worn.
After measuring, pinned specimens were rehydrated in either distilled or deionised water for approximately 24 hours and then dissected to assess ovary development (OD; Richards et al. Reference Richards, Vickruck and Rehan2010; Corbin et al. Reference Corbin, Awde and Richards2021). Females lacking developed ovaries were assigned the score OD = 0, and those possessing thickened ovaries with no visible oocytes were assigned the score OD = 0.1. For females with visible, developing oocytes, each oocyte was assigned a proportional score of 0.25, 0.5, 0.75, or 1, with 1 representing a fully developed oocyte (Richards et al. Reference Richards, Vickruck and Rehan2010). These scores were then summed, producing a total ovary development score for each female. Any female which possessed at least one oocyte of at least 1/2 size (0.5) was considered “fecund” (Breed Reference Breed1976).
Females collected before male emergence were categorised as P1, and those collected after male emergence were assigned to P2. P1 females were presumed to be overwintered nest foundresses produced in the previous year. P2 females with total wear scores of less than 1 (TW ≤ 1) and ovary development scores of 0 (OD = 0) were assumed to be newly eclosed brood 2 daughters that had left their nests to mate and feed before diapause; these were not included in trait comparisons between P1 and P2 females. All other females were assumed to be foragers collecting brood provisions (Richards et al. Reference Richards, Vickruck and Rehan2010; Corbin et al. Reference Corbin, Awde and Richards2021). Foundress longevity was roughly estimated based on the proportions of worn females collected during P2, as these likely were foundresses that had survived past P1.
Statistical analyses
All statistical analyses were conducted using R, version 3.6.2 (https://www.r-project.org/), running under R-Studio (https://rstudio.com), version 1.2.5033, and were based on comparisons of foraging females collected in phases 1 and 2. For body comparisons of Ontario bees, which were collected in multiple years, we initially checked for annual variation in size using a general linear model of the form Head width ∼ Phase × Year, but the Year term was not significant. We used t-tests for size comparisons for both Ontario and Alberta bees. For comparisons of total wear and total ovary development of phase 1 and 2 females, we used Kruskal–Wallis tests, a nonparametric equivalent of analysis of variance. To compare the proportions of worn and fecund females in phases 1 and 2, we used Pearson’s X 2-tests with Yates’ continuity correction.
Results
Lasioglossum leucozonium in southern Ontario
The flight season of Niagara L. leucozonium lasted about 23 weeks (Table 2). The phenology was univoltine (Fig. 3). Males and newly eclosed females were first collected during week 13 in early July, which was considered to mark the beginning of P2. The majority of females (n = 153, 82%) were collected during P1, but worn females (n = 33, 18%) were collected as late as week 23 (late September). Twelve fecund females with high wear scores (largest oocyte ≥ 0.5 and TW > 5) were collected during P2, including one female collected in week 19 (end of August) that had a fully developed oocyte ready to lay. This indicates that a few very long-lived foundresses were still laying eggs near the end of the flight season. Large numbers of males were collected in September, peaking in week 23.

Figure 3. The univoltine phenology of L. leucozonium in Ontario, based on specimens collected from 2003 to 2018. The vertical grey line marks the end of P1 and beginning of P2, based on the first appearance of males in week 13.
P1 and P2 females were of similar head width (t = 0.667, df = 42.183, nonsignificant; Fig. 4), as expected if they represented a single cohort. The proportions of worn females were similar in P1 (130/149 = 87%) and P2 (31/33 = 94%, X 2 = 0.620, df = 1, nonsignificant); however, the median wear score was significantly higher in P2 (H = 5.46, df = 1, P = 0.019; Fig. 4). The proportions of fecund females did not significantly differ in P1 (125/153 = 82%) and P2 (22/33 = 67%, X 2 = 0.620, df = 1, nonsignificant); however, ovary development scores were significantly lower in P2 (H = 4.54, df = 1, P = 0.033; Fig. 4), suggesting that old foundresses laid eggs at a lower rate.

Figure 4. Reproductive traits of female sweat bees collected in P1 (before first appearance of males) and P2 (after first appearance of males). Full statistical results are reported in the text. The larger body size and higher ovary development scores of P2 females in L. zonulum from Ontario are a unique pattern in bivoltine sweat bees.
Lasioglossum zonulum in southern Ontario
The flight season of Niagara L. zonulum lasted 20 weeks from early May to early September (Table 2; Fig. 5). Two major periods of foraging activity were observed, separated by a slight hiatus in activity during weeks 9–10, indicating that this population is bivoltine. Newly eclosed females from brood 1 were first collected in early July during week 12, whereas the first males were collected in week 13; because the appearance of newly eclosed females preceded that of males, week 12 was taken as the beginning of P2. P1 females were deemed to be nest foundresses, whereas P2 females likely represented a mix of old foundresses (F0), brood 1 (F1) daughters provisioning brood 2, and newly eclosed brood 2 daughters (F2) foraging before hibernation. Approximately 55% (n = 137) of foragers were collected in P1, and approximately 45% (n = 111) were from P2.

Figure 5. The bivoltine phenology of L. zonulum in Niagara, Ontario, based on 954 specimens collected from 2003 to 2018. Note the distinct decline in captures of females in weeks 9–12. The vertical grey line marks the end of P1 and beginning of P2, based on the first appearance of males in week 12.
P2 females were significantly larger than P1 females, both overall (t = –2.50, df = 243.15, P = 0.013; Fig. 4) and in 11 of the 12 years in which females were collected in both phases. The proportion of worn females rose from P1 (108/137 = 79%) to P2 (101/111 = 91%, X 2 = 5.95, df = 1, P = 0.0147), and the median wear score was significantly higher in P2 (H = 47.36, df = 1, P < 5.91e-12; Fig. 4). The proportion of fecund females also rose from P1 (68/137 = 50%) to P2 (93/111 = 84%, X 2 = 29.92, df = 1, P = 4.51e-08), and ovary development scores were significantly higher in P2 (H = 18.36, df = 1, P = 1.82e-05; Fig. 4).
Lasioglossum zonulum in southern Alberta
Female bees were recorded from week 9 in mid-June until week 19, suggesting a 10-week flight season ending in late August (Table 2). However, most females (79%) were collected early, in weeks 9–11 (Fig. 6). The large number of females caught in week 9 suggests that the flight season may have begun before the first collections, but the shape of Figure 6 is consistent with univoltinism. The first males were collected in week 16, in early August, only four weeks before the end of the flight season. Because collection week for Calgary was calculated using the median dates of roughly week-long collection events, we designated week 15 (late July) as the beginning of phase 2 (Table 2). Using this date, 79% (n = 108) foundresses were collected during P1, and 21% (n = 28) were collected in P2. The latter represent long-lived females that survived and provisioned brood right up to the end of the flight season. One single, newly eclosed female (TW = 0, OD = 0) was collected in late August (week 19), indicating that brood emergence continued until the end of the summer.

Figure 6. The univoltine phenology of Calgary L. zonulum in 2016. For specimens collected in Alberta, a collection refers to all the bees trapped at a single location over a period of 5–14 days, with “week” designating the mid-point of a collection period. Captures of males are emphasised with small arrows; males comprised only 1.5% (n = 13) of specimens and were collected only after week 16.
As predicted for a single cohort of foundresses, P1 and P2 females were similar in size (t = 0.738, df = 41.350, nonsignificant; Fig. 4). The proportions of worn females were similar in the two phases (P1: 101/108 = 94%, P2: 28/28 = 100%, X 2 = 0.816, df = 1, nonsignificant), but P2 females had markedly higher wear scores (H = 48.157, df = 1, P = 3.93e-12; Fig. 4). The proportions of fecund females did not differ significantly between the two phases (P1: 58/98 = 59%, P2: 10/28 = 32%, X 2 = 0.816, df = 1, nonsignificant), but ovary development scores were significantly lower during phase 2 (H = 10.116, df = 1, P = 0.00147; Fig. 4).
Discussion
Our first goal in this study was to provide detailed phenological and social information for three Canadian populations of L. leucozonium and L. zonulum. For L. leucozonium, we observed univoltine solitary behaviour in southern Ontario, whereas for L. zonulum, we observed bivoltine solitary behaviour in Ontario and univoltine solitary behaviour in Alberta. Combined with the somewhat sparse information available from other regions (Table 1), our findings suggest that L. leucozonium is obligately univoltine and solitary throughout its range, whereas L. zonulum is phenologically flexible but obligately solitary, being univoltine in Alberta where breeding seasons are shorter and bivoltine solitary in Ontario where breeding seasons are longer. Our second goal was to compare details of solitary reproduction in univoltine and bivoltine populations, which could help to increase understanding of the kinds of behavioural trait changes that might occur in forward or reverse transitions between solitary and eusocial behaviour (Fig. 1).
Traits of univoltine solitary sweat bees
Two populations, Ontario L. leucozonium and Alberta L. zonulum, were univoltine solitary; the similarities between them perhaps represent reproductive traits typical of univoltine solitary sweat bees. In both populations, most females were collected in P1, before the emergence of males, suggesting that most nest foundresses were short-lived and died before their offspring emerged as adults. However, a few, very worn females were collected in P2, suggesting that some long-lived foundresses were still engaged in provisioning activity months after the first brood in the population had reached adulthood. In Ontario L. leucozonium, 17.7% of foundresses survived to P2, so the longest-lived foundresses might have produced offspring over a period as long as five months (May to September; Table 2). This suggests they could have raised many more offspring than the 6–15 suggested by Pesenko et al. (Reference Pesenko, Banaszak, Radchenko and Cierzniak2000). In Alberta L. zonulum, 7.4% of all females and 20.6% of females measured and dissected were collected in P2; despite the less precise collection dates, this suggests that old foundresses were still provisioning brood late in the breeding season, several weeks after the oldest brood emerged. Perhaps it is characteristic of univoltine solitary sweat bees that most foundresses have short lives, provisioning brood for perhaps eight weeks in spring and early summer, whereas a minority are long-lived and have reproductive lifespans as long as 4–5 months.
Additional similarities between univoltine populations of L. leucozonium and L. zonulum were found in female traits. In both univoltine populations, P1 and P2 females were similar in size, but P2 females were more worn and had less ovary development. Because all foragers would have been members of the same foundress cohort, the similarity in size suggests size-independent mortality. The accumulating wear and declining ovary development suggest that, in general, fertility declines with age, even though some females have long reproductive lifespans. These univoltine solitary bees seem to fit the definition of indeterminate breeders, initiating brood production in spring and continuing to provision and lay eggs until senescence and morbidity catch up with them (Kennedy Reference Kennedy1991). In indeterminate breeders, the major predictor of maternal reproductive success is maternal longevity: the longer a foundress lives, the more brood cells she provisions and eggs she can lay (Bosch and Vicens Reference Bosch and Vicens2006).
The long lifespans of some univoltine foundresses may create opportunities for behavioural interactions among foundresses and their adult daughters. Egg-to-adult development time in groundnesting sweat bees varies from about 3 to 6 weeks (Kocher et al. Reference Kocher, Pellissier, Veller, Purcell, Nowak, Chapuisat and Pierce2014), whereas flight seasons last up to 20 weeks, suggesting that a substantial minority of univoltine foundresses could live long enough to encounter their adult offspring inside the nest. Behavioural evidence for interactions between mothers and offspring in solitary sweat bees is mixed. In two species that likely are obligately solitary, L. (E.) villosulum (Kirby) and L. (L.) quadrinotatum (Kirby), mothers ignore their offspring after eclosion, whereas in some facultatively solitary/eusocial species, mothers do interact with brood (Plateaux-Quénu Reference Plateaux-Quénu2008). Behavioural interactions between foundresses and their newly eclosed daughters might influence behaviours relevant to understanding social flexibility. For instance, mothers might influence whether daughters overwinter in their natal nests or elsewhere, which in turn could influence the probability of communal nesting, because daughters that overwinter in the same nest might be more likely to remain together in spring (Packer and Knerer Reference Packer and Knerer1985; Richards and Packer Reference Richards and Packer1998).
Traits of bivoltine solitary sweat bees
Based on our observation that L. zonulum is bivoltine in Ontario but univoltine in Alberta, we can now add it to the list of facultatively bivoltine species in Lasioglossum (Leuchalictus) (Table 1). Facultative bivoltinism implies flexibility in diapause timing (Hunt et al. Reference Hunt, Kensinger, Kossuth, Henshaw, Norberg, Wolschin and Amdam2007; Santos et al. Reference Santos, Arias and Kapheim2019), whereas geographic variation in phenology suggests that diapause decisions are influenced by environmental cues such as changes in daylength (Gill et al. Reference Gill, Gaurav and Gurminder2017). Our results suggest that in Alberta, L. zonulum daughters enter overwintering diapause soon after adult eclosion, whereas in Ontario, brood 1 daughters commence brood production. Our data do not allow us to rule out the possibility that the Ontario population is actually partially bivoltine, with some brood 1 females entering diapause while others commence reproduction. Partial bivoltinism underlies social polymorphism in Halictus rubicundus Christ, in which some brood 1 females become workers in the maternal nest while others enter diapause and become nest foundresses the following spring (Yanega Reference Yanega1988). Detecting partial bivoltinism in Ontario, L. zonulum would require behavioural observations of marked females produced in brood 1 in order to observe how many commence brood provisioning and how many reappear as nest foundresses the following spring (Yanega Reference Yanega1988).
The clear hiatus in foraging activity of Ontario L. zonulum between P1 and P2 was a distinctive feature of the observed bivoltine phenology. This hiatus could reflect high foundress mortality in weeks 9–12 (Fig. 5), but L. leucozonium foragers from the same sites do not decline in abundance until week 11 (Fig. 3). An alternative explanation is that L. zonulum foundresses are determinate breeders that stop laying eggs once a predetermined clutch size has been reached (Kennedy Reference Kennedy1991) and then close their nests and remain inside until their brood 1 daughters reach adulthood. It is noteworthy that eusocial sweat bees also exhibit an extended hiatus in foraging activity between P1 and P2 (Corbin et al. Reference Corbin, Awde and Richards2021), although the timing of the hiatus varies among species. Eusocial queens provision a small number of brood, then cease brood provisioning, close their nests until the first workers reach adulthood, and rarely leave their nests after workers begin foraging. In the current study, we collected very worn P2 females that may have been P1 foundresses that had resumed foraging after the hiatus. These could have been foraging alongside their brood 1 daughters – again, nest-based observations are needed to investigate this contrast with eusocial species.
The significantly larger sizes and higher ovary development scores of P2 females in Niagara L. zonulum seem to be unique observations for bivoltine sweat bees, in which foundresses are generally larger than their daughters. For instance, in two solitary species, L. (Hemihalictus)villosulum (Kirby) and L. (Dialictus) ellisiae (Sandhouse), foundresses are about 7.4% and 3.5% larger than their daughters, respectively (Plateaux-Quénu et al. Reference Plateaux-Quénu, Plateaux and Packer1989; Corbin et al. Reference Corbin, Awde and Richards2021). Size differences of this magnitude are on par with queen–worker size differences regularly observed in eusocial sweat bees (Breed Reference Breed1976; Packer and Knerer Reference Packer and Knerer1985; Awde and Richards Reference Awde and Richards2018). A possible proximate explanation for this unique observation is that relative body sizes of P1 and P2 females reflect the availability of pollen resources during P2 (when P1 foundresses are provisioned) compared to during P1 (when P2 daughters are provisioned). Evidence supporting this hypothesis comes from detailed foraging observations of a solitary carpenter bee, Ceratina calcarata Robertson, found at some of the same sites where we collected L. zonulum. Ceratina females make longer foraging trips and smaller-sized brood (Rehan and Richards Reference Rehan and Richards2010; Lewis and Richards Reference Lewis and Richards2017) as the flight season progresses, suggesting that access to pollen resources declines over the summer. If pollen availability is higher when P2 daughters are provisioned, this could explain the larger size of P2 females. However, higher pollen availability in P1 should also result in P1 females laying more eggs (Peterson and Roitberg Reference Peterson and Roitberg2006). P1 females had lower, not higher, ovary development than P2 females did.
The combination of larger size and greater egg-laying capacity in P2 compared to P1 females suggests an alternative explanation. In female bees, larger body size is associated with higher fecundity, longer flight distances, and more efficient foraging (Bosch and Vicens Reference Bosch and Vicens2006; Rehan and Richards Reference Rehan and Richards2010; Zurbuchen et al. Reference Zurbuchen, Landert, Klaiber, Müller, Hein and Dorn2010), so larger size would be especially advantageous for females that provision brood during phase 2 if floral resources are more difficult to access. Thus, P1 females that provision brood when resources are plentiful should invest in daughters larger than themselves because those daughters would benefit greatly from the larger investment in size. Testing this hypothesis would require measurements of P1 and P2 females in additional L. zonulum populations, but if similar patterns were observed across populations, it could be argued that it is not the large size of L. zonulum daughters that begs explanation, but the small size of daughters in other bivoltine solitary species.
Potential biases in trapping studies
Most of our specimens were collected in pan traps or blue vane traps. All bee-collecting methods are subject to biases, in the sense that certain taxa tend to be predictably under- or over-represented according to the collection method. Traps certainly vary in their attractiveness to different kinds of bees, to females and males, and as the surrounding vegetation changes (Cane et al. Reference Cane, Minckley and Kervin2000; Tuell and Isaacs Reference Tuell and Isaacs2009; Baum and Wallen Reference Baum and Wallen2011), issues could emerge that, if unrecognised, might lead to biased estimates of local bee species composition, density, or relative abundance. However, direct comparisons of phenological and behavioural inferences based on specimens collected via trapping or nest-based observations produce substantially similar and unbiased results when mass-trapped specimens are used to infer flight phenology and to provide female specimens for the inspection of reproductive traits (Richards et al. Reference Richards, Vickruck and Rehan2010, Reference Richards, Onuferko and Rehan2015; Corbin et al. Reference Corbin, Awde and Richards2021). That multiple studies have demonstrated equivalent outcomes should alleviate concerns about their utility for inferences about phenology or female reproductive traits. Analyses of trapped specimens cannot substitute for nuanced behavioural observations of individually marked females, the gold standard for studies of bee social behaviour, but they are valuable for facilitating behavioural inferences that would otherwise not be possible in the absence of nest observations.
Conclusions
Sweat bees have long been regarded as an ideal taxon for studying the evolution of eusociality. Perhaps because solitary behaviour is frequently regarded as “simple” and because biologists have been beguiled by the complexity of sociality, the breeding behaviour of solitary sweat bees has been largely neglected (M.H. Richards, D.A. Skandalis, P. Davison, S. Kocher, unpublished data). Yet understanding solitary behaviour is a necessary prerequisite for reconstructing the sequences of evolutionary changes that occur when eusociality evolves, as well as the changes that happen when eusocial lineages revert to solitary behaviour. By studying solitary sweat bees, we may yet discover whether reversals to solitary behaviour recapitulate solitary-to-eusocial steps in reverse or result in subtle behavioural differences between ancestrally and secondarily solitary species. A more detailed understanding of solitary reproduction will also help us to better understand the costs and benefits of solitary versus group reproduction in different circumstances.
Acknowledgements
The authors are grateful to Paul Galpern for providing specimens from Calgary and to Lincoln Best for identifying them. They also thank members of the Brock Bee Lab for their help and encouragement, especially while A.P. finished his thesis during the COVID pandemic, and the reviewers for their helpful comments.











