Article contents
SPATIAL DISTRIBUTION AND SEQUENTIAL SAMPLING METHODS FOR THE POTATO APHID, MACROSIPHUM EUPHORBIAE (THOMAS) (HOMOPTERA: APHIDIDAE), IN OILSEED FLAX1
Published online by Cambridge University Press: 31 May 2012
Abstract
Sequential decision plans based on aphid counts and binomial counts of infested plants (presence or absence of aphids) were developed to guide chemical control decisions for die potato aphid, Macrosiphum euphorbiae, on two growth stages of oilseed flax in western Canada. The plans were derived from studies of aphid dispersion among plants in field plots at two locations over 4 years, and verified in samples from 51 commercial fields, in Manitoba. The relationship between variance (s2) and mean aphid density (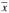 ) perplant was loges2 = 0.790 ± 0.050 + (1.649 ± 0.031) loge
) perplant was loges2 = 0.790 ± 0.050 + (1.649 ± 0.031) loge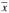 (n = 69, r2 = 0.98), for both crop growth stages. Neither sweep samples nor pan samples produced reliable estimates of the number of aphids per plant and, therefore, these sampling tools could not replace aphid counts on individual plants. Aphid counts and the binomial method gave similar control decisions with similar amounts of effort, but the aphid counting method required fewer plants to reach a decision. The same decisions were reached in 85–95% of fields by counting aphids on a minimum sample of 25 plants when the crop was in full bloom, or 20 plants at the green boll stage, as with samples of 50–100 plants.
(n = 69, r2 = 0.98), for both crop growth stages. Neither sweep samples nor pan samples produced reliable estimates of the number of aphids per plant and, therefore, these sampling tools could not replace aphid counts on individual plants. Aphid counts and the binomial method gave similar control decisions with similar amounts of effort, but the aphid counting method required fewer plants to reach a decision. The same decisions were reached in 85–95% of fields by counting aphids on a minimum sample of 25 plants when the crop was in full bloom, or 20 plants at the green boll stage, as with samples of 50–100 plants.
Résumé
Les stratégies d’échantillonnage séquentiel basées sur l’abondance des pucerons ou sur les données présence- absence dans les plants infestés ont servie à élaborer des politiques de lutte chimique du Puceron de la pomme de terre, Macrosiphum euphorbiae, dans des champs de lin de l’ouest du Canada, à deux stades de croissance. Les stratégies ont été mises au point à partir d’études de la dispersion des pucerons dans les plants dans des carrés échantillons sur une période de 4 ans, puis essayées dans 51 cultures commerciales au Manitoba. La relation entre la variance (s2) et la densité moyenne des pucerons (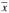 ) par plant se traduit par l’équation suivante loges2 = 0,790 ± 0,050 + (1,649 ± 0,031) loge
) par plant se traduit par l’équation suivante loges2 = 0,790 ± 0,050 + (1,649 ± 0,031) loge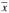 (n = 69, r2 = 0,98), aux deux stades de croissance des cultures. Ni les échantillons recueillis au filet fauchoir, ni ceux recueillis dans des bacs n’ont permis d’obtenir des estimations fiables du nombre de pucerons par plant et ces outils ne peuvent donc pas remplacer le dénombrement exact des pucerons dans un plant particulier. L’abondance des pucerons et les données présence–absence on donné lieu à des décisions semblables requérant des efforts de même importance, mais la méthode du dénombrement exact permet d’arriver aux décisions en utilisant moins de plants. Les mêmes conclusions ont été obtenues dans 85–95% des champs en comptant les pucerons sur un échantillon minimum de 25 plants au stade de la floraison, de 20 plants au stade de capsule verte, ou sur des échantillons de 50–100 plants ne tenant pas compte du stade.
(n = 69, r2 = 0,98), aux deux stades de croissance des cultures. Ni les échantillons recueillis au filet fauchoir, ni ceux recueillis dans des bacs n’ont permis d’obtenir des estimations fiables du nombre de pucerons par plant et ces outils ne peuvent donc pas remplacer le dénombrement exact des pucerons dans un plant particulier. L’abondance des pucerons et les données présence–absence on donné lieu à des décisions semblables requérant des efforts de même importance, mais la méthode du dénombrement exact permet d’arriver aux décisions en utilisant moins de plants. Les mêmes conclusions ont été obtenues dans 85–95% des champs en comptant les pucerons sur un échantillon minimum de 25 plants au stade de la floraison, de 20 plants au stade de capsule verte, ou sur des échantillons de 50–100 plants ne tenant pas compte du stade.
[Traduit par la Rédaction]
- Type
- Articles
- Information
- Copyright
- Copyright © Entomological Society of Canada 1995
References
- 6
- Cited by


