Introduction
The genus Cinara Curtis is the most speciose of the Lachninae subfamily (Hemiptera: Aphididae), with more than 250 valid species (Favret Reference Favret2023). Of those, approximately 50 are found in East Asia, but many more may exist that have not yet been described (Chen et al. Reference Chen, Jiang, Chen and Qiao2016b). Lachninae originally evolved on woody angiosperms, but Cinara diversified dramatically when its ancestor adopted a coniferous host (Chen et al. Reference Chen, Favret, Jiang, Wang and Qiao2016a). This genus is characterised by a medium to large body, a short antennal processus terminalis, a rostrum with a distinct joint between the fourth and fifth segments, and pigmented cone-shaped siphunculi (Eastop Reference Eastop1972).
In their Reference Eastop, Miyazaki and Sorin1998 publication, Eastop et al. listed 31 species of Cinara occurring in Japan and provided an identification key that included five species not formally described. Collections made in Japan in 2010 recovered two populations of their “Cinara sp. C” on Abies homolepis Siebold and Zuccarini (Pinaceae). Following an evaluation of these new specimens and those used by Eastop et al. (Reference Eastop, Miyazaki and Sorin1998) from Abies firma Siebold and Zuccarini, we confirmed that they do indeed represent a new species and here describe it as such. Along with a morphological description, we provide a molecular diagnosis that satisfies Article 13.1.1 of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature 1999).
Aphids were hand-collected in the field, preserved in 95% ethanol, and kept at –80 °C. A nondestructive protocol (Favret Reference Favret2005) was used to extract DNA from two specimens per population using a DNeasy Blood and Tissue Kit (Qiagen, Düsseldorf, Germany). The cleared aphid cuticles were then slide-mounted in Canada balsam (Favret Reference Favret2005). The identification keys of Inouye (Reference Inouye1970), Eastop et al. (Reference Eastop, Miyazaki and Sorin1998), and Blackman and Eastop (Reference Blackman and Eastop1994, Reference Blackman and Eastop2022) were used for specimen determination. When available in the Ouellet-Robert Entomological Collection of the Université de Montréal (QMOR; Montréal, Québec, Canada), specimens of Japanese Cinara and those found on Abies spp. worldwide were compared directly. The original published species descriptions were used as a comparative reference when actual material was unavailable and as a source for specimen measurements.
Specimens are deposited at: (1) the National Agriculture and Food Research Organisation, Tsukuba, Japan (NARO); (2) the aphid collection of the Smithsonian Institution National Museum of Natural History, Beltsville, Maryland, United States of America (USNM-ENT); and (3) the Université de Montréal Ouellet-Robert Entomological Collection (QMOR). Specimen data are published by Hébert et al. (Reference Hébert, Sano and Favret2023) and from there are available at the Global Biodiversity Information Facility (GBIF). Catalogue numbers with decimals refer to individual specimens on a slide that has more than one specimen. Specifically, NARO-210102729.001 and NARO-210102729.002 refer to two individual specimens on slide NARO-210102729.
Polymerase chain reaction amplification of the 5´ region of the mitochondrial cytochrome c oxidase subunit 1 (CO1) gene was realised using LepF and LepR primers (Foottit et al. Reference Foottit, Maw, von Dohlen and Hebert2008) and standard protocols (Théry et al. Reference Théry, Kanturski and Favret2018). The 608- and 660-nucleotide sequences are deposited and available at GenBank (accession numbers: OP536219 and OP536220). To see if any molecular autapomorphies were present in our new species, the alignments of our sequences with those of Cinara species known from Japan and those having Abies spp. as hosts (but not necessarily restricted to Japan) were realised using the Clustal Omega alignment in Geneious (Sievers et al. Reference Sievers, Wilm, Dineen, Gibson, Karplus and Li2011). Some sequences were taken from GenBank (accession numbers: GU978789, KP339517, KM501334, JX034927, KP339747, KR031213, GU978840, JQ916795, and KC201787), and others come from our personal libraries. We recorded the nucleotide substitutions unique to the two species based on their positions when aligned with a reference sequence from the Acyrthosiphon pisum (Harris) (Aphididae: Macrosiphini) mitochondrial genome (accession number: FJ411411).
Z-stacked photographs were taken in brightfield lighting, except for Figure 3A, which was photographed under differential interference contrast conditions, with a Zeiss M2 AxioImager microscope, an AxioCam HRc camera, and Zen 2012 Software, version 1.1.1.0 (Carl Zeiss AG, Oberkochen, Germany).
The morphological abbreviations are as follows: BL – body length (measured from the frontal margin of the head to the end of the cauda); BW – greatest body width; HWE – head width at eyes; ANT I, II, III, IV, V, VI – antennal segments I, II, III, IV, V, VI, or their length; PT – processus terminalis of the last antennal segment or its length; BASE – base of the last antennal segment or its length; LSANT III – length of the longest setae on antennal segment III; ROS – rostrum or the length of the sclerotised stylet groove; ROS IV, V – fourth or fifth segment of the rostrum or their length; URS – ultimate rostral segments (IV + V) or their length; HCOX – width of the hind coxa; LMF – length of the metafemur; WMF – largest width of the metafemur; LMT – length of the metatibia; WMT – largest width of the metatibia; LDMTS – length of the longest dorsal setae of the metatibia; LVMTS – length of the longest ventral setae of the metatibia; HT I – first segment of the hind tarsus or its length (measured on the ventral side); HT II – second segment of the hind tarsus or its length; ABD III – third segment of the abdomen; SIPH – siphunculus or its diameter at base; and SPIR – spiracular sclerites or their diameters.
A single measurement per character was made on each specimen. It may have been of either the left or right of the specimen, depending on which side had the more visible and easily measured character. The mean diameter of the spiracular sclerites did not statistically differ between each abdominal segment (F 6,63, P = 0.249); therefore, the measures given represent the mean and range of the seven pairs of abdominal spiracles of the combined specimens. All measurements are in micrometres (μm).
Cinara stigmatica sp. nov
ZooBank Nomenclature Act: urn:lsid:zoobank.org:act:3BA802A2-E7F4–46C9-AC44-A992C5F0B802
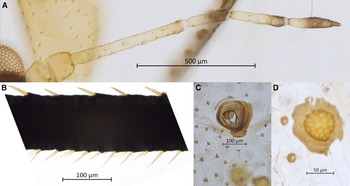
Holotype. NARO-210104343 (Fig. 1): apterous vivipara, Japan, Nagano Prefecture, Fujimi-kôgen Highland, 35.9370° N, 138.3176° E ± 10m, 2010–07–03, on Abies homolepis, Colin FAVRET and Masakazu SANO leg. Paratypes. NARO-210102729, 2 apterous viviparae, Japan, Miyagi Prefecture, Kinkasan, [38.294° N, 141.565° E ± 2500 m], 1966–08–05, on Abies firma, Zenpei YAMASHITA leg.; USNM-ENT760076, QMOR71646, QMOR71649, QMOR71650, QMOR71636, five apterous viviparae, Japan, Yamanashi Prefecture, Yamanaka-ko Lake, 35.4469° N, 138.8547° E ± 10 m, 2010–07–02, on Abies homolepis, Colin FAVRET and Masakazu SANO leg.; QMOR71647, QMOR71648, 2 apterous viviparae, Japan, Nagano Prefecture, Fujimi-kôgen Highland, 35.9370° N, 138.3176° E, 2010–07–03, on Abies homolepis, Colin FAVRET and Masakazu SANO leg. See also Hébert et al. (Reference Hébert, Sano and Favret2023).

Figure 1. Holotype of Cinara stigmatica sp. nov. (NARO-210104343): A, holotype slide; and B, habitus of apterous vivipara holotype specimen.
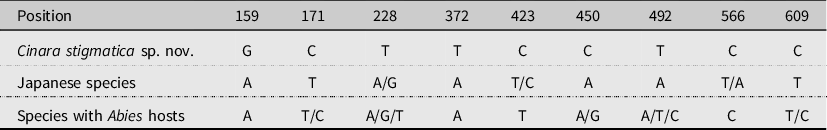
Diagnosis. The new species can be distinguished by a combination of characters: (1) the size and colour of the SPIR, (2) the colour of the tibiae, (3) the length of the tibial setae, and (4) the appearance of the muscle attachment plates. It shares some of those characters and its host associations with C. matsumurana Hille Ris Lambers, 1966 (sensu Inouye Reference Inouye1970), C. todocola (Inouye, 1936) (sensu Inouye Reference Inouye1970), and C. longipennis (Matsumura, 1917) (sensu Inouye Reference Inouye1970). It differs in that the length of the dorsal tibial setae is much shorter – 54.82 μm (208.62 μm in C. longipennis, 171.74 μm in C. matsumurana, and 118.43 μm in C. todocola) – and all tibiae are all black (lighter apical region in C. matsumurana and basal region in C. longipennis). The main difference between this new species and C. todocola is the presence of large spinal and marginal sclerites on the dorsum of the abdomen in the latter. It is also morphologically close to C. formosana (Takahashi, 1924) (sensu Inouye Reference Inouye1970) in having short and thick setae, prominent muscle attachment plates surrounded by sclerites, and in the size and colour of the SPIR, but it differs in that HT II is 2.57–2.98 × HT I (1.6–1.9 in C. formosana). There are also no scleroites at the base of the setae in C. formosana, their tibiae are lighter in colour, and it is known to feed on Pinus spp. The keys to the Cinara spp. on Abies spp. of Blackman and Eastop (Reference Blackman and Eastop1994, Reference Blackman and Eastop2022) lead to C. kiusa Hottes, 1957 (couplets 25 and 31 in the 1994 and 2022 versions, respectively), but the ROS is much shorter in our species, reaching only to the HCOX (reaching past the SIPH in C. kiusa). For DNA characters, eight diagnostic nucleotides were found when compared with species known from Japan and four were found when compared with those that have Abies spp. as a plant host (Table 1).
Table 1. Diagnostic nucleotide differences in CO1 gene between Cinara stigmatica sp. nov., 23 Japanese Cinara species, and 21 Cinara species that have Abies spp. as hosts.
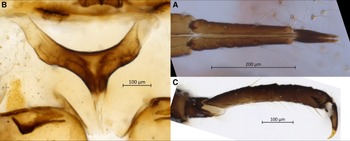
Colour. In life: green. In slide-mounted specimens: head and body beige (Fig. 1B). Antennae pale (Fig. 2A), with darker apical half on ANT IV–VI. Coxae, SIPH, abdominal sclerotisation, genital plate, anal plate, and cauda yellowish to light brown. Femora yellowish brown, with hind femur dark brown on distal half to three-quarters and front and middle femur sometimes with a darker line on the posterior face. Tibiae all black (Figs. 1B and 2B). SPIR dark brown (Fig. 2C). Muscle attachment plates light to dark brown (Fig. 2D).
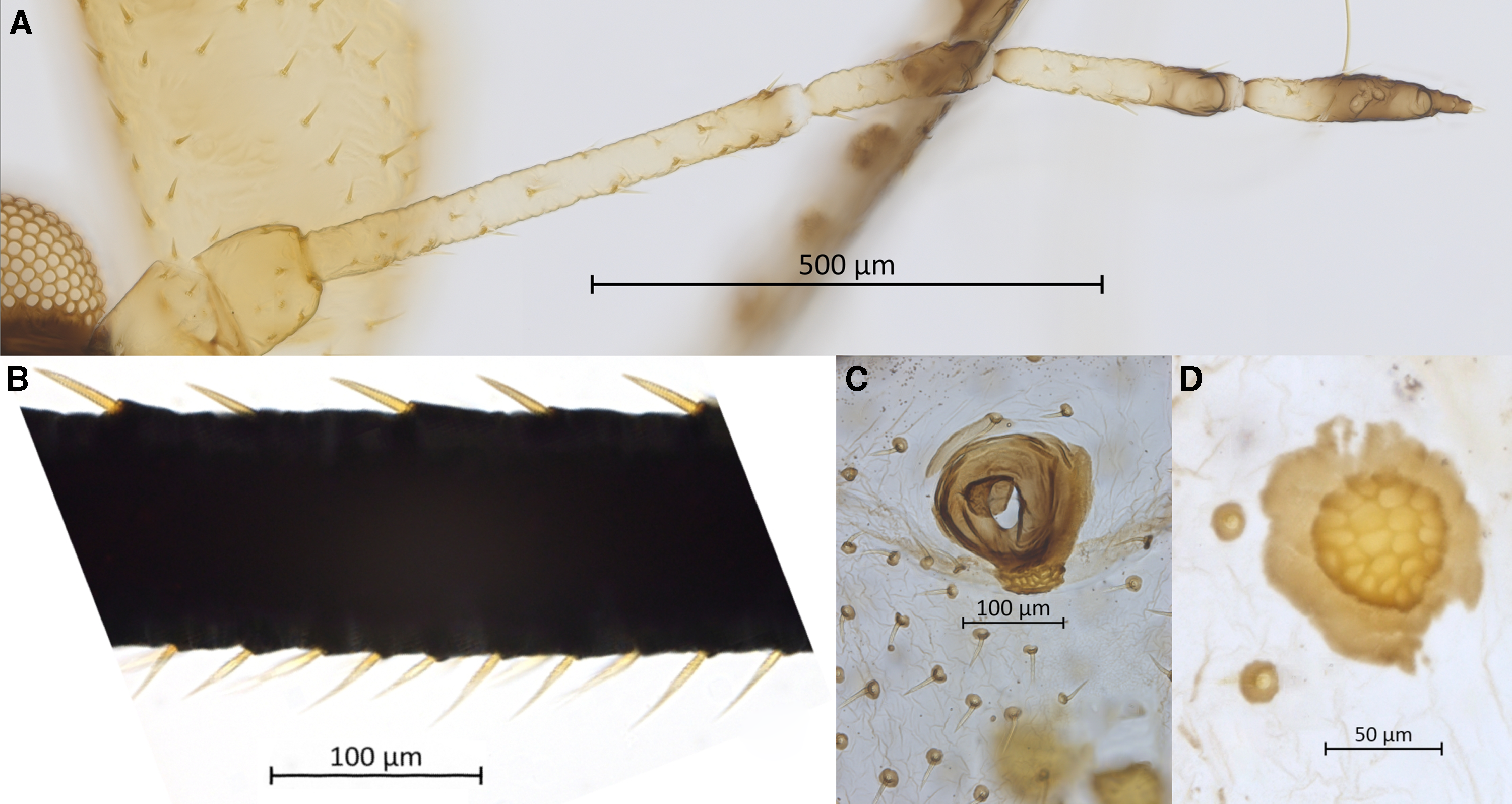
Figure 2. Morphological features of apterous vivipara of Cinara stigmatica sp. nov.: A, antenna (NARO-210104343); B, dorsal (upper) and ventral (lower) hind tibial setae. (NARO-210104343); C, large spiracular sclerite with adjoined muscle attachment plate and ventral setae with scleroites (QMOR71647); D, large sclerotised muscle insertion plate and dorsal setae with scleroites (NARO-210104343).
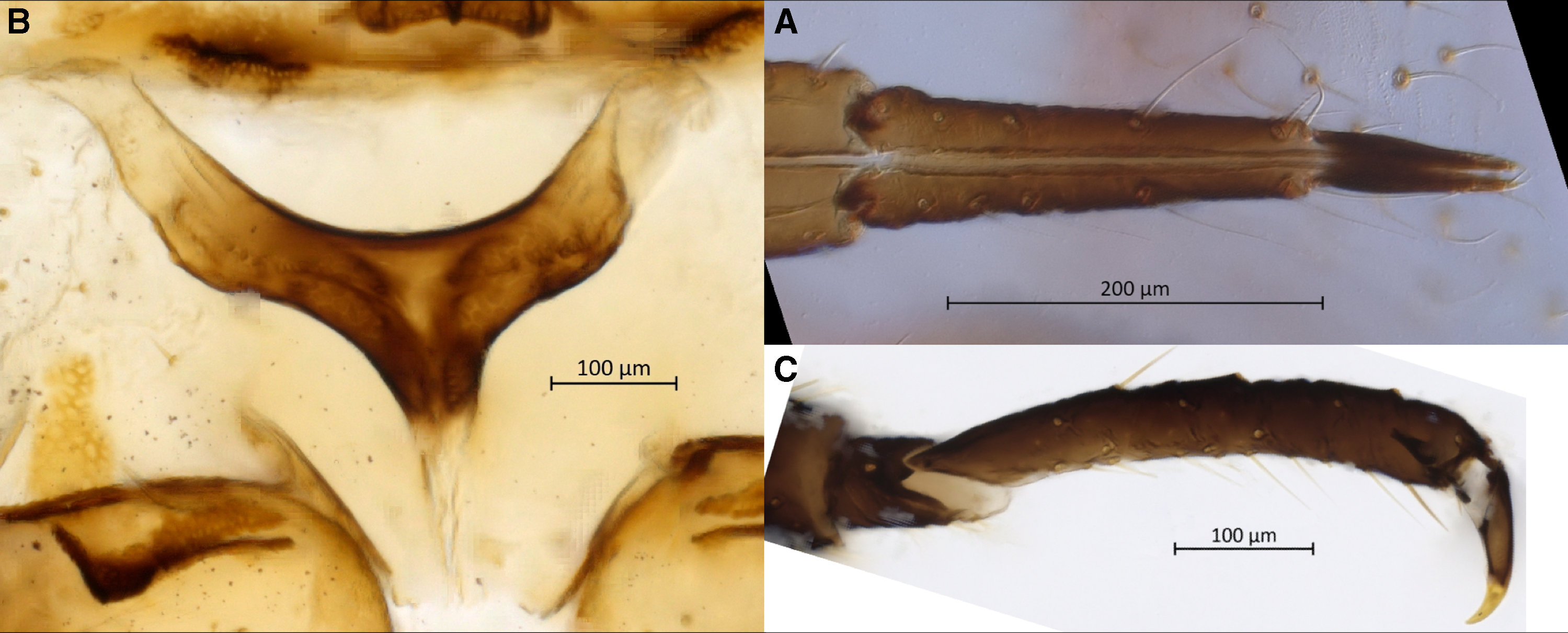
Figure 3. Morphological features of apterous vivipara of Cinara stigmatica sp. nov.: A, ultimate rostral segments bearing seven accessory setae (NARO-210102729.002); B, mesosternal furca (QMOR71647); C, hind tarsus (USNM-ENT760076).
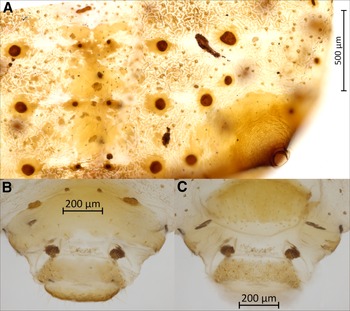
Body. Large aphid (BL 3969–4685 µm) with an oval-shaped body (Fig. 1B). HWE 0.42–0.54 × ANT. ANT 0.28–0.32 × BL. Head. Head covered by pointed setae. Triommatidium distinct. ANT III longest, 0.52–0.75 × SIPH and without secondary sensoria. ANT IV shortest and without secondary sensoria. ANT V 0.94–1.33 × ANT VI and 0.44–0.56 × HT II, with one rounded secondary sensorium. ANT VI with a cluster of approximately four small secondary sensoria laterad to the primary one, with PT 0.21–0.39 × BASE and bearing four subapical setae. Antennae with short and spinelike setae. LSANT III 0.73–0.90 × the width of ANT III. ROS reaching the HCOX, 0.33–0.42 × BL. ROS IV 1.96–2.28 × ROS V and 1.51–1.77 × HT I, bearing four to eight accessory setae (Fig. 3A). Thorax. Mesosternal furca fused, Y-shaped with short arms and a well-developed stem (Fig. 3B). Prosternal tubercle distinct. Femora and coxae large. HCOX 1.59–2.06 × WMF. Femora and tibiae with short and spinelike setae (Fig. 2B). LMT 39.58–51.76 × LDMTS. HT II with few dorsal and more numerous ventral short spine-like setae (Fig. 3C), 1.66–1.82 × ROS IV. Abdomen. Dorsal and ventral setae with small scleroites (Figs. 2C and D). Ventral setae on ABD III finer and longer (44.82–68.14 μm) than the dorsal ones (30.42–46.68 μm). Dorsum, especially posteriorly, sometimes textured with irregularly shaped darkened patterns (lighter or darker in different specimens; Fig. 4A). Small and irregular spinal sclerites on tergites (III) IV–VI (Figs. 1B and 4A). Large rounded SPIR with adjoined muscle insertion plate (Fig. 2C), 0.21–0.30 × SIPH. Muscle insertion plates distinct and often surrounded by a lighter sclerotic region on the dorsum (Figs. 2D and 4A). SIPH broadly conical with a large, pigmented area at the base (Fig. 4A), 4.59–8.89 × SIPH pore. Eighth abdominal tergite bearing 14–24 setae (Fig. 4B). Genital plate with 59–77 setae (Fig. 4C). Cauda and anal plate spinulose, bearing long and fine setae. Cauda semicircular (Fig. 4B). Measurements are given in Table 2.
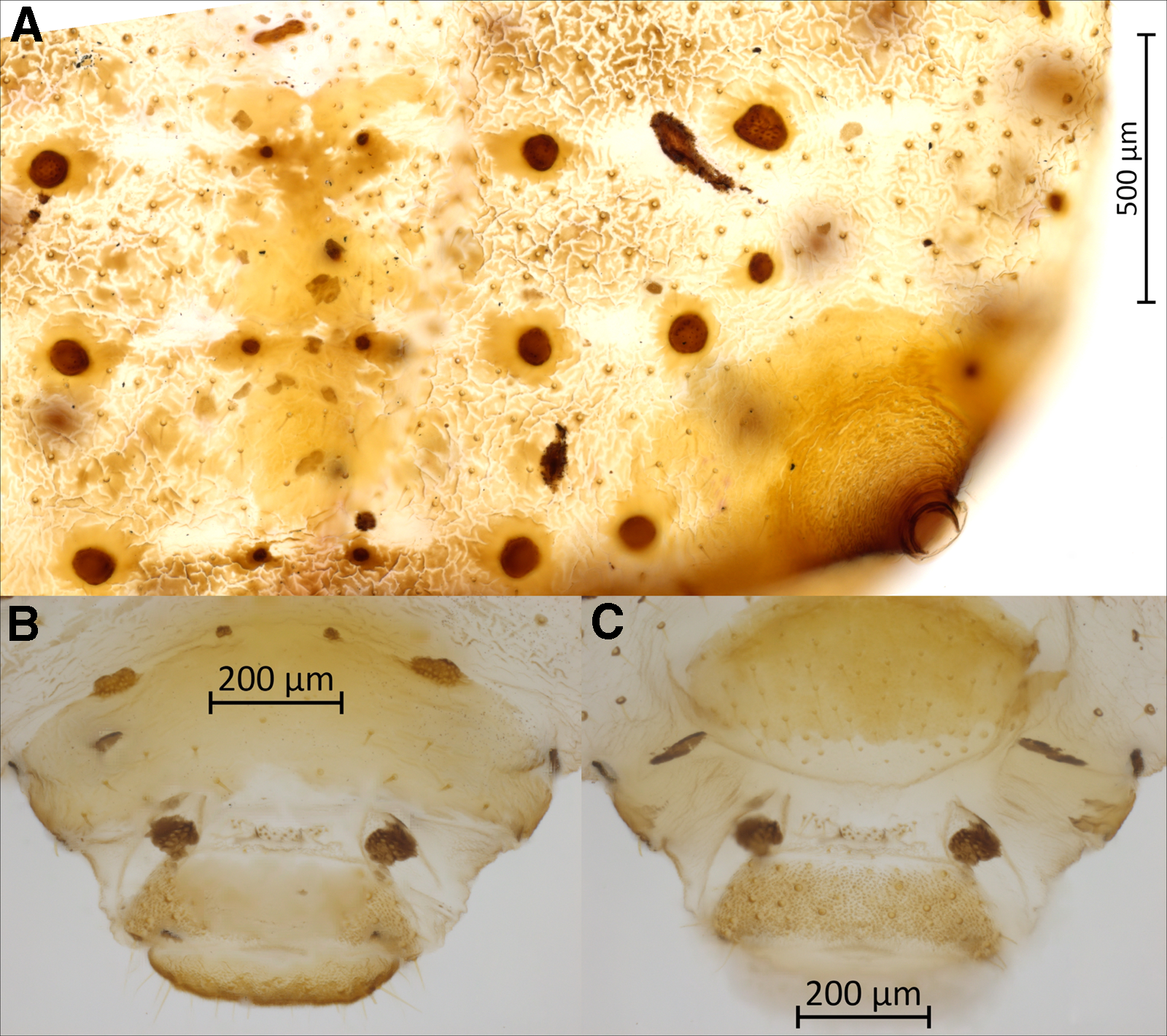
Figure 4. Morphological features of apterous vivipara of Cinara stigmatica sp. nov.: A, dorsal abdominal segments IV, V, and VI, bearing spinal sclerites, three rows of muscle attachment plates, pigmented patterning, setae with scleroites, and siphunculus with broad sclerotic base and numerous setae visible as pale spots throughout its surface (NARO-210102729.001); B, dorsum of terminal abdominal segments; the scale bar is placed over the eighth abdominal tergite; below that are two muscle attachment plates on either side of the rudimentary gonapophyses, followed by the anal plate and cauda (QMOR71647); C, venter of terminal abdominal segments; the scale bar is placed over the cauda; above that is the anal plate, followed by two muscle attachment plates on either side of the rudimentary gonapophyses, and above these the genital plate (QMOR71647).
Table 2. Measurements (in μm) of apterous viviparous females of Cinara stigmatica sp. nov.
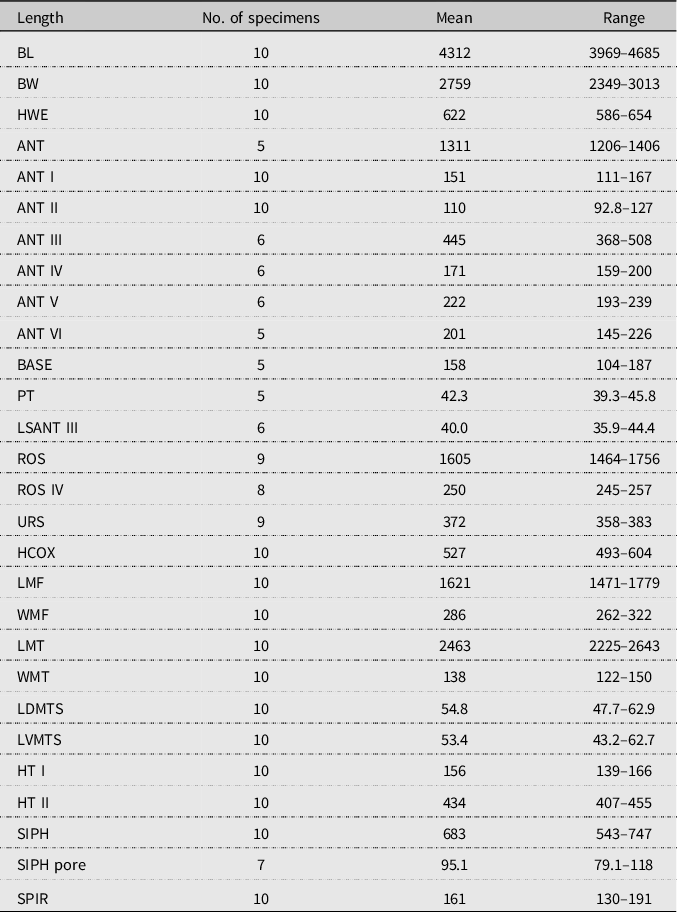
BL, body length; BW, greatest body width; HWE, head width at eyes; ANT I, II, III, IV, V, VI, antennal segments I, II, III, IV, V, VI or their length; PT, processus terminalis of the last antennal segment or its length; BASE, base of the last antennal segment or its length; LSANT III, length of the longest setae on antennal segment III; ROS, rostrum or the length of the sclerotised stylet groove; ROS IV, V, fourth or fifth segment of the rostrum or its length; URS, ultimate rostral segments (IV + V) or their length; HCOX, width of the hind coxa; LMF, length of the metafemur; WMF, largest width of the metafemur; LMT, length of the metatibia; WMT, largest width of the metatibia; LDMTS, length of the longest dorsal setae of the metatibia; LVMTS, length of the longest ventral setae of the metatibia; HT I, first segment of the hind tarsus or its length; HT II, second segment of the hind tarsus or its length; ABD III, third segment of the abdomen; SIPH, siphunculi or its diameter at base; SPIR, spiracular sclerites or their diameters.
Etymology. The species is named for the sclerotic spiracles (from “stigmate”, the French term for spiracle). The feminine ending for this adjective accords with the grammatically feminine Cinara; the masculine and neuter forms are stigmaticus and stigmaticum, respectively.
Molecular analysis. A BLAST search on GenBank with our CO1 sequences returned a closest match of 94% similarity, much farther than the 2% dissimilarity threshold that is commonly used to discriminate animal species (Hebert et al. Reference Hebert, Ratnasingham and de Waard2003) or the 2% found to be the average intraspecific divergence in Cinara species (Chen et al. Reference Chen, Jiang and Qiao2012). The single close genetic match in the Barcode of Life Data System (BOLD) was 98.6% (BOLD 2023). The single specimen (BIOUG00939-C10), from Changbai Mountain in Jilin Province, China, likely is Cinara stigmatica sp. nov., but we are unable to evaluate it properly as it is an immature unassociated with any host.
Specimen data publication. Data accessibility is lacking in classical taxonomy. Although specimen metadata are always presented, most of the information about the material examined is in simple text format within the article. Specimen data availability in machine-readable format on open-access repositories such as GBIF can contribute to subsequent research (Favret Reference Favret2014; Gemeinholzer et al. Reference Gemeinholzer, Vences, Beszteri, Bruy, Felden and Kostadinov2020). According to Heberling et al. (Reference Heberling, Miller, Noesgaard, Weingart and Schigel2021), the use of data from GBIF in scientific research has increased, whereas specimen-based data deposited at GBIF from explicitly taxonomic resources have decreased. There is great potential for taxonomists to help the field and others make use of the valuable information linked to every specimen observed in their work: the “material examined” specimen data published in taxonomic works are typically of the highest quality and, in our opinion, their accessibility in a machine-readable format should be a matter of course. Sheffield and Heron (Reference Sheffield and Heron2018) and Nelson and Moffat (Reference Nelson and Moffat2022) published, at Canadensys.net, downloadable Darwin Core archives (Sheffield Reference Sheffield2018; Nelson Reference Nelson2023) to accompany their respective regular articles. We added to their model by publishing a data paper that is as citable as any other journal article (Costello et al. Reference Costello, Michener, Gahegan, Zhang and Bourne2013; Hébert et al. Reference Hébert, Sano and Favret2023). We hope this new model will incentivise taxonomists to better share their valuable specimen data.
Acknowledgements
The authors thank two anonymous and unremunerated reviewers for their precious time and advice. They also thank Yukinobu Nakatani (Institute for Plant Protection, NARO, Tsukuba, Japan) and Mariusz Kanturski (Institute of Biology, University of Silesia, Katowice, Poland) for access to the original (1966) specimens, and Shin-ichi Akimoto (Hokkaido University) for help during Favret’s visit to Hokkaido University. The authors acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC) for an Undergraduate Student Research Award to Hébert and a Discovery Grant to Favret (RGPIN 2017-06287), and that of the Global Center of Excellence Program (Establishment of the Center for Integrated Field Environmental Science) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, for an Invitation Fellowship Program for Overseas Researcher award to Sano.
Competing interests
Apart from their current and future employment and funding, the authors declare no conflicts of interest.