Introduction
The Rocky Mountain wood tick, Dermacentor andersoni Stiles, and the American dog tick, Dermacentor variabilis (Say) (Acari: Ixodidae), require three hosts to complete their life cycles, and they feed on many of the same species of mammalian hosts. Larvae and nymphs feed on small mammals (e.g., meadow voles, deer mice, western jumping mice, and red squirrels), whereas adults feed on medium- to large-sized mammals (e.g., raccoons, dogs, white-tailed deer, and cattle; Wilkinson Reference Wilkinson1984; Dergousoff et al. Reference Dergousoff, Galloway, Lindsay, Curry and Chilton2013; Lindquist et al. Reference Lindquist, Galloway, Artsob, Lindsay, Drebot, Wood and Robbins2016). Although D. andersoni and D. variabilis have largely allopatric ranges in western Canada, they coexist in a zone of sympatry in southwestern Saskatchewan (Dergousoff et al. Reference Dergousoff, Galloway, Lindsay, Curry and Chilton2013), where they experience the same off-host environmental conditions. Nonetheless, the two species differ significantly in their phenology (Lindquist et al. Reference Lindquist, Galloway, Artsob, Lindsay, Drebot, Wood and Robbins2016). In western Canada, the larvae and, to a lesser extent, the adults of D. variabilis overwinter (Yunik et al. Reference Yunik, Galloway and Lindsay2015; Diyes et al. Reference Diyes, Dergousoff, Yunik and Chilton2021), whereas nymphs and adults are the overwintering stages for D. andersoni (Wilkinson Reference Wilkinson1984). In this study, we describe the differences in the behaviour of D. variabilis and D. andersoni larvae after emergence from eggs and how this relates to the differences observed in the phenology of their life cycles.
The larvae used in this study were the progeny of questing adult ticks collected in the field during June 2018 and June 2019. The collection site for D. variabilis in both years was Lizard Lake Community Pasture (52.405, −107.912), located approximately 50 km north of Biggar, Saskatchewan, whereas D. andersoni were collected in 2019 in an area adjacent to Chin Lakes Reservoir (49.643, −112.208) in Alberta, Canada. Conspecific male and female ticks were attached to Canadian Arcott sheep as described in Diyes et al. (Reference Diyes, Dergousoff, Yunik and Chilton2021). Different sheep were used as hosts for the two tick species. Egg clutches were obtained from 28 engorged D. variabilis females (16 in 2018 and 12 in 2019) and 12 engorged D. andersoni females (all in 2019) that had been placed individually into sterile glass tubes (21 × 65 mm) capped with perforated rubber stoppers. These vials were maintained at a constant 25 °C, 95% relative humidity, and in darkness (Fig. 1). A saturated potassium nitrate (KNO3) solution was used to maintain the 95% relative humidity (Winston and Bates Reference Winston and Bates1960). Females started laying eggs 2–7 days after completing the blood meal, in late June to early July. On each day of the oviposition cycle, all eggs laid by a female tick (= an egg pool) were carefully transferred into a sterile plastic 0.5-mL tube with a perforated lid and maintained under the same constant environmental conditions as the ovipositing females (Fig. 1). Observations were made daily to determine when eggs in each pool began to hatch (= day 1 on Fig. 1). Two days after hatching, the newly emerged larvae from each egg pool were transferred into a new sterile plastic 0.5-mL tube with a perforated lid. A fine nylon mesh was placed inside the lid of each tube to prevent larval escape. Tubes containing larvae were kept in darkness at 25 °C and 95% relative humidity. Larvae in each tube were observed for movement over 2–3 minutes using a STEMI SV 8 Zeiss microscope (Oberkochen, Germany) without a light source. In 2018, the behaviour of the D. variabilis larvae derived from 278 egg pools (i.e., comprising 11–887 larvae per pool) was observed daily for a maximum of 321 days. In 2019, daily observations were made on the behaviour of 230 pools (7–979 larvae per pool) of D. andersoni larvae and 201 pools (9–744 larvae per pool) of D. variabilis larvae (Fig. 1). Observations in 2019 ended when the mortality rate reached 90% for D. andersoni or by 65 days for D. variabilis (Fig. 1). In experiments conducted in 2018 to determine the survival times of D. variabilis larvae (Diyes et al. Reference Diyes, Dergousoff, Yunik and Chilton2021) and D. andersoni larvae in 2019, some larval pools were transferred on day 49 or 52 (D. andersoni and D. variabilis, respectively; Fig. 1) to one of 15 combinations of three constant temperatures (5 °C, 25 °C, and 32 °C) and five relative humidities (35, 55, 75, 85, and 95%). All larvae were kept in darkness, and observations were made daily of larval behaviour during these experiments.
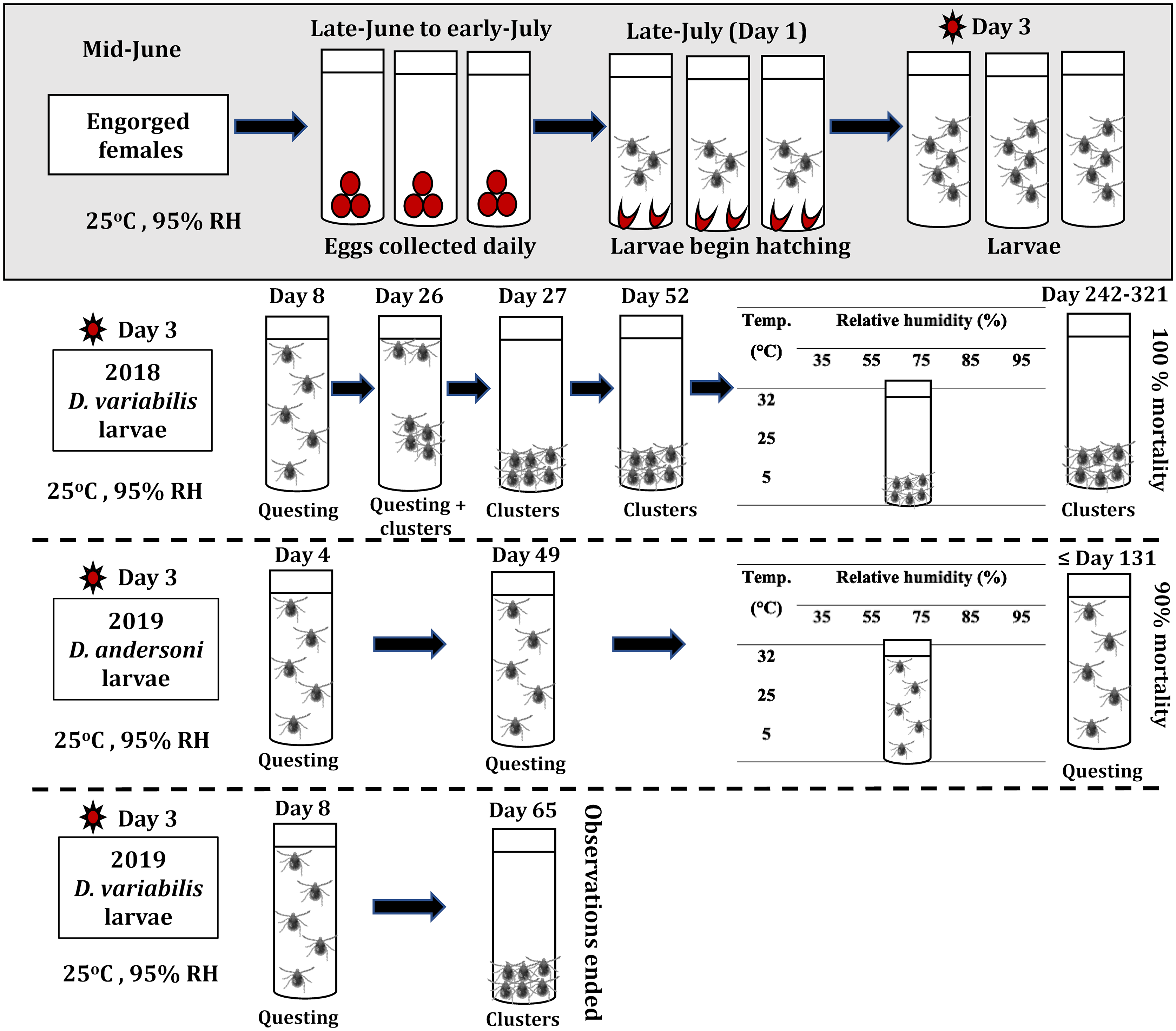
Figure 1. The experimental procedure used for obtaining Dermacentor variabilis and Dermacentor andersoni larvae and assessing their behaviour and mortality under different laboratory conditions. Dermacentor variabilis larvae displayed questing activity and clustering while D. andersoni larvae displayed continuous questing activity until their death. RH, relative humidity.
After hatching from the eggs, both D. variabilis and D. andersoni larvae climbed and aggregated on the side wall of each tube (Fig. 1). During this stage, the translucent larvae were motionless with their legs tucked beneath their bodies, and they did not engage in any questing activity for several days. This period of inactivity corresponds to the period during which there is sclerotisation of the tick cuticle (i.e., a maturation phase in the life cycle), which ranges for 4–7 days in other tick species (Gladney et al. Reference Gladney, Drummond, Whetstone and Ernst1970; Davey Reference Davey1986). However, differences between the two tick species were observed when host-seeking activity commenced and in the duration of this questing behaviour. For D. andersoni, larvae began questing on day 4 (Fig. 1). These larvae moved upwards and downwards within the tube (Fig. 2), and this movement was interspersed with the clustering of larvae at the top of the tube. In this position, larvae remained motionless except for the outstretching of their forelegs. This behaviour was observed in the larvae produced by all 12 D. andersoni females. Dermacentor andersoni larvae continued their questing activity, regardless of the environmental conditions to which they were exposed, until death – a maximum of 131 days at 25 °C and 95% relative humidity and 114 days at 5 °C and 95% relative humidity.
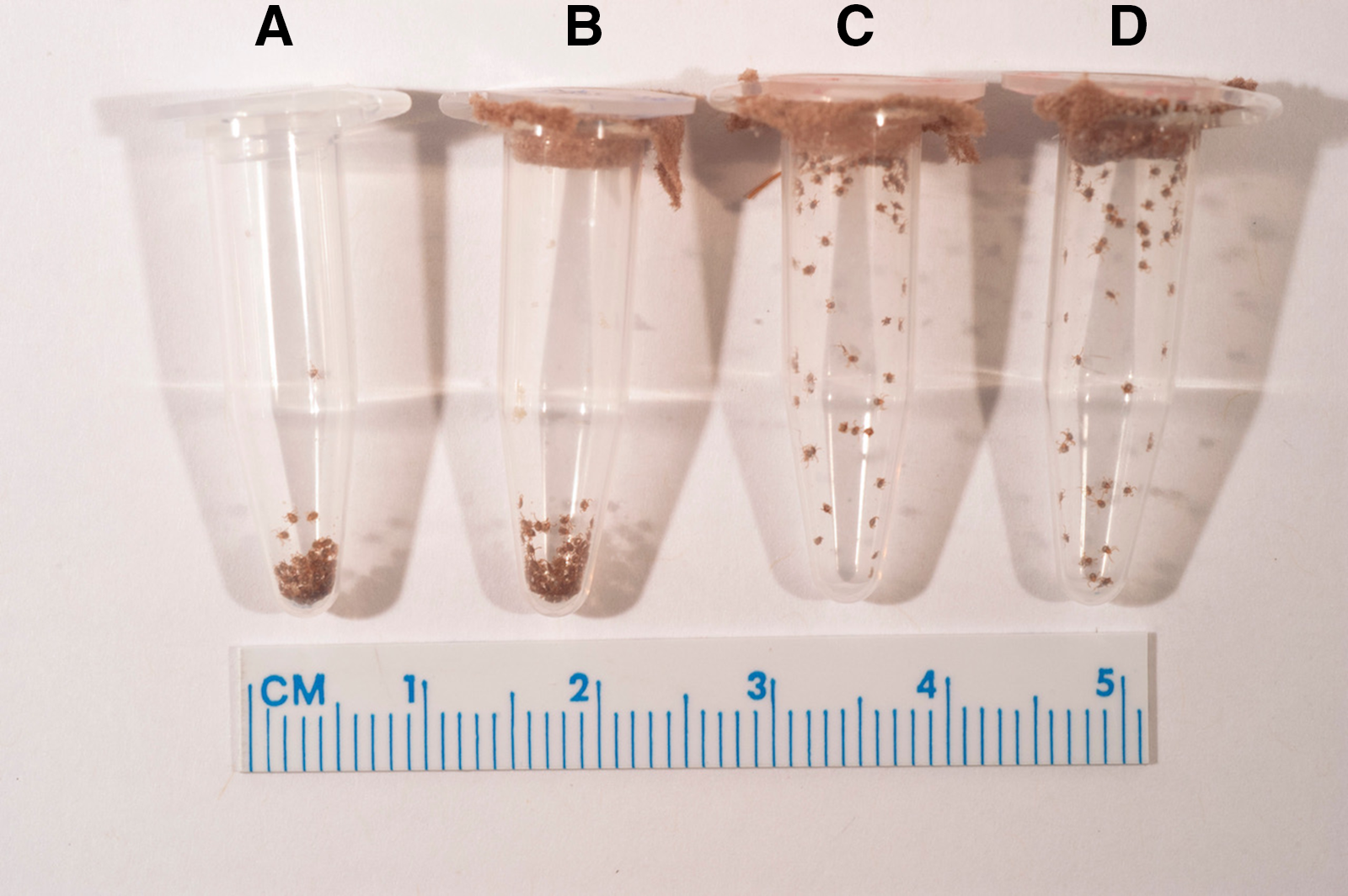
Figure 2. Representative image of the activity of Dermacentor variabilis and Dermacentor andersoni larvae 106 days posthatch. A and B, D. variabilis larvae showing clustering activity/larval aggregations at the bottom of each tube; C and D, dispersed D. andersoni larvae showing questing behaviour.
For D. variabilis, larvae began questing on day 8, and their behaviour was the same as that seen for D. andersoni larvae. However, on day 25, some D. variabilis larvae continued questing and others began to aggregate on the side walls of the tubes. By day 26, all larvae from the 16 D. variabilis females (in 2018) formed clusters and remained motionless at the bottom of the tubes. The same behaviour was observed for the larvae produced by the 12 D. variabilis females in 2019 (Fig. 1). Moreover, the larvae kept under different environmental conditions also maintained their behavioural diapause. This behavioural diapause for D. variabilis larvae continued until their death or the termination of the experiment. Dermacentor variabilis larvae in 2018 ceased engaging in questing activity for a maximum of 294 days (from 18 August 2018 to 7 June 2019). Behavioural diapause in ticks is either obligatory, which is determined genetically, or facultative, which is controlled by environmental cues, such as changes in photoperiod (Belozerov Reference Belozerov2009; Leal et al. Reference Leal, Zamora, Fuentes, Thomas and Dearth2020). Smith and Cole (Reference Smith and Cole1941) stated that daylength was an important factor in controlling the initiation of diapause in D. variabilis. However, in the present study, larvae were maintained in darkness, which suggests that the initiation of behavioural diapause in D. variabilis larvae is not determined by environmental cues but possibly by an innate mechanism (i.e., a biological clock). Thus, the factors influencing behavioural diapause in D. variabilis require further investigation. Furthermore, given the conditions of the present experiments, no photoperiodic cues occurred to trigger a termination of the diapause in the D. variabilis larvae, especially for those larvae that survived for more than 200 days. In addition, cessation of behavioural diapause in unfed D. variabilis nymphs is triggered by increasing temperatures (Belozerov Reference Belozerov2009); however, we found that transferring D. variabilis larvae to different temperature and relative humidity regimes did not induce questing. Photoperiod, perhaps in combination with increasing temperature, is important to terminate the behavioural diapause and induce questing in D. variabilis larvae.
A marked interspecific difference was observed in the behaviour of larvae exposed to the same environmental conditions. The absence of a behavioural diapause in D. andersoni larvae is consistent with their seasonal patterns of activity (Wilkinson Reference Wilkinson1968, Reference Wilkinson1984) and inability to survive off-host from summer, over winter, through to the next spring (Wilkinson Reference Wilkinson1968; Sonenshine et al. Reference Sonenshine, Yunker, Clifford, Clark and Rudbach1976; Owen et al. Reference Owen, Vander Vliet and Scoles2014). Dermacentor andersoni larvae begin attaching to small mammal hosts in late June, and this activity peaks in July and August and then gradually declines in September (Sonenshine et al. Reference Sonenshine, Yunker, Clifford, Clark and Rudbach1976; Wilkinson Reference Wilkinson1984). In a montane population (i.e., collected from Kamloops, British Columbia, Canada), this larval activity extends from May to October (Wilkinson Reference Wilkinson1968). Unfed D. andersoni larvae are less tolerant to water loss than D. variabilis larvae are (Knülle Reference Knülle1966; Diyes et al. Reference Diyes, Dergousoff, Yunik and Chilton2021) and are unable to survive more than 10 weeks in summer (Sonenshine et al. Reference Sonenshine, Yunker, Clifford, Clark and Rudbach1976). This limited off-host survival is consistent with the results of laboratory experiments that show that D. andersoni larvae survive for only 3–7 days at constant temperatures of 25 °C and 32 °C and up to 75% relative humidity. Therefore, given these physiological limitations, questing D. andersoni larvae must find a suitable host as soon as possible after emerging from the eggs and the hardening of their cuticle.
In contrast, field experiments conducted from summer through to early spring at a site close to Lizard Lake Community Pasture (i.e., near the northern distributional limit of this species) have demonstrated that unfed D. variabilis larvae have a very high (95%) survival rate (Diyes et al. Reference Diyes, Dergousoff, Yunik and Chilton2021). This finding would explain the presence of D. variabilis larvae on small mammals in March to May in southern Canada (Garvie et al. Reference Garvie, Mckiel, Sonenshine and Campbell1978). A second peak of larval activity on hosts also occurs from July to August (Garvie et al. Reference Garvie, Mckiel, Sonenshine and Campbell1978; Burachynsky and Galloway Reference Burachynsky and Galloway1985). In our lab experiments, eggs were laid in late June/early July, and the majority of D. variabilis larvae emerged by late July, which would be equivalent to the second larval peak on hosts in the field. Interestingly, termination of the questing behaviour of D. variabilis larvae in the lab occurred on day 27, which corresponded to mid-August, close to the last time of the year when larvae are found on small mammal hosts (Burachynsky and Galloway Reference Burachynsky and Galloway1985). The cessation of questing resulted in clustering of D. variabilis larvae at the bottom of the tubes and would represent an adaptation (e.g., reduce their metabolic expenditure and rate of water loss; Fielden and Lighton Reference Fielden and Lighton1996; Yoder and Knapp Reference Yoder and Knapp1999; Leal et al. Reference Leal, Zamora, Fuentes, Thomas and Dearth2020) in the field to successfully survive off-host from late summer through to the subsequent spring. Dermacentor variabilis larvae retain water more effectively when present in clusters (Yoder and Knapp Reference Yoder and Knapp1999) and survive longer in moisture-rich environments (Knülle Reference Knülle1966; Sonenshine Reference Sonenshine and Rodriguez1979; Diyes et al. Reference Diyes, Dergousoff, Yunik and Chilton2021). Under laboratory conditions, D. variabilis larvae produced by the adults collected from Lizard Lake Community Pasture survived for more than 100 days at constant temperatures of 25 °C and 32 °C and at least 85% relative humidity (Diyes et al. Reference Diyes, Dergousoff, Yunik and Chilton2021).
In summary, the behavioural differences displayed by newly emerged larvae of D. variabilis and D. andersoni occurred despite being exposed to the same environmental conditions. The continuous host-seeking activity of D. andersoni larvae and a long diapause in D. variabilis larvae followed by a questing phase are consistent with their respective phenologies. This study represents the first published account of a difference in the behaviour of newly emerged D. andersoni and D. variabilis larvae produced by females collected from populations at the northern parts of the species’ distributional ranges. The behavioural diapause exhibited by D. variabilis larvae may represent an important adaptation that is contributing to the northward range expansion by this species; however, this warrants further investigation.
Acknowledgements
The National Science and Engineering Research Council of Canada (NSERC Discovery Grant to NBC) and the University of Saskatchewan (Dean’s Scholarship and University Graduate Scholarship to CPD) provided funding for this study. The authors thank Marlynn Mierau for his assistance with Figure 2.
Competing interests
The authors declare they have no competing interests.





