Introduction
Cluster flies, or Pollenia Robineau-Desvoidy, 1830, are a commonly observed genus of true flies (Diptera: Polleniidae), readily distinguished by their dull-coloured bodies with golden setae on the thorax (Whitworth Reference Whitworth2006). They are often disregarded beyond their role as house pests, and a quick search of their common name would produce endless results instructing how to rid your home of them. This view has remained unchanged throughout their history in North America, with one early account being typical, suggesting that “words fail to describe their general depravity; it is beyond expression. If you wish to be happy, be sure you don’t introduce cluster flies into your family” (Dall Reference Dall1882). These plentiful flies often cluster together on walls, ceilings, and windows, entering any cracks in walls and roofs of houses to overwinter (Oldroyd Reference Oldroyd1964).
Several hundred flies may gather in any one cluster (Lintner Reference Lintner1893), and in early spring, hundreds of flies may die in homes before they are able to find their way back outside (Shewell Reference Shewell1987). Aside from inside houses, Pollenia have been found overwintering in leaf sheaths of corn stalks, under bark (DeCoursey Reference DeCoursey1927), in trees using old woodborer galleries (Dennys Reference Dennys1927), in the abandoned galleries of darkling beetles in rotting hoof fungi (Will Reference Will1995), and in a bald-faced hornet (Hymenoptera: Vespidae) nest (Greenberg Reference Greenberg1998). The flies are abundant in the spring and fall but are rarely noticed in the summer by the general public (DeCoursey Reference DeCoursey1927).
Pollenia are widely regarded as harmless nuisance pests (Goble Reference Goble1972; Allen-McGill Reference Allen-McGill1983). Despite this, there is evidence that these flies could spread bacterial pathogens (Thomson Reference Thomson1972; Faulde et al. Reference Faulde, Sobe, Burghardt and Wermter2001), the accumulation of dead flies can cause allergies, and their corpses may act as breeding material for dermestid beetles and other pests (Spencer Reference Spencer1928; Shewell Reference Shewell1987). Pollenia are suspected to have contributed to one case of faecal coliform bacterial contamination in a water reservoir in Martinborough, New Zealand, where massive numbers of the flies were present in and on the reservoir (Heath et al. Reference Heath, Marris and Harris2004). In addition to spreading bacteria, spores of Entomophthora schizophorae S. Keller and Wilding, 1988 (Entomophthoraceae) are known to persist within Pollenia and to transmit between flies as they overwinter in unheated attics (Eilenberg et al. Reference Eilenberg, Thomsen and Jensen2013).
History in North America: 1800s
It is unknown exactly when Pollenia were first introduced to North America (Gisondi et al. Reference Gisondi, Rognes, Badano, Pape and Cerretti2020). If all North American Pollenia species are found to be obligate earthworm parasites, cluster flies likely arrived when earthworms were introduced to North America by European settlers as early as the 1500s (Reynolds Reference Reynolds1995), limiting the arrival of Pollenia to an approximate window of 350 years. Howard (Reference Howard1911) stated that the date of introduction to the United States of America was unknown. Pollenia were first reported in North America between 1820 and 1822 from Nova Scotia (Walker Reference Walker1849; Piers Reference Piers1917). More records soon followed: 1858 (Osten-Sacken 1858) and again in 1862 (Loew Reference Loew1862), although no specific localities were reported in either case. The next Canadian reference appears to be from 1867, with specimens collected in Québec (van der Wulp Reference van der Wulp1867). Lintner (Reference Lintner1893) recalled seeing flies in 1875 in Schenectady, New York, United States of America that he believed to be Pollenia, although he did not know how to classify them at the time of observation. Osten-Sacken (Reference Osten-Sacken1878) included this record in an 1878 revision of his 1858 report, documenting the species Pollenia rudis (Fabricius, 1794). These specimens were, at least at the time, housed in the collection of Diptera of the Museum of Comparative Zoology in Cambridge, Massachusetts, United States of America (1878), but any Pollenia specimens belonging to this collector (Osten-Sacken) that currently remain in the collection could not be distinguished (C. Maier, personal communication). As such, modern species classifications for the Pollenia specimens in Osten-Sacken’s publications could not be determined.
In 1882, Dall wrote about specimens of Pollenia he had received from a relative in Geneva, New York, in what appears to be the first North American reference to Pollenia as household pests (Dall Reference Dall1882). That relative recalled “it is probably thirty years since the flies appeared in our neighbourhood” (Dall Reference Dall1882), which puts their arrival somewhere in the early 1850s by their account, within the same decade as Osten-Sacken’s (1858) publication. The flies were described as sluggish, cold, and oily, and as existing in the country, with few occurring in towns and villages (Dall Reference Dall1882). They were reportedly hard to kill, unlike other house pests (Dall Reference Dall1882), even when pyrethrum powder was used (Mann Reference Mann1882); however, other authors reported that this method was successful (Lintner Reference Lintner1893). These reports from the late 1800s include Québec, Canada (van der Wulp Reference van der Wulp1867) and New England, Washington (Dall Reference Dall1882), New Jersey (Smith Reference Smith1890), and New York, United States of America (Dall Reference Dall1882; Lintner Reference Lintner1893).
History in Canada: 1900s
Canadian reports of Pollenia continued in the 1900s. In 1928, Pollenia were described as household pests that caused “considerable alarm” in Ontario (Ross and Caesar Reference Ross and Caesar1928). Although the journal volume in question (the 95th Annual Report of the Entomological Society of Ontario, 1928) contains articles with species lists from Prince Edward Island, Nova Scotia, New Brunswick, Quebec, Manitoba, Saskatchewan, Alberta, and British Columbia, Canada, Pollenia are mentioned only in the Ontario paper (Ross and Caesar Reference Ross and Caesar1928). Interestingly, they were excluded in a subsequent publication by Caesar (Reference Caesar1941). For many years, the Annual Reports of the Entomological Society of Ontario published summaries of pest insects. A series by C.G. MacNay from 1950 to 1960 (Volumes 81–91) reported on Canadian infestations in which Pollenia were mentioned from 1951 onwards as a common household pest in Ontario and Québec. Other provinces, including British Columbia (MacNay Reference MacNay1953, Reference MacNay1954), Alberta (MacNay Reference MacNay1951), and the Gulf of St. Lawrence provinces (MacNay Reference MacNay1953), had less-consistent reports. None were found in the Prairie provinces (MacNay Reference MacNay1953). A similar series by W.C. Allan from 1967 (Allan Reference Allan1967) to 1973 (Volumes 98–104) focussed exclusively on Ontario and stated that the flies were abundant pests each year.
Taxonomy
Despite the classifications of several species of Pollenia in Europe, nearly all of the early accounts of this genus in North America reported specimens as P. rudis (Cranshaw and Due Reference Cranshaw and Due2018). Aldrich (Reference Aldrich1905) listed three species, at least one of which is synonymous with P. rudis; however, the other species names seem to have been largely disregarded in subsequent publications. Rognes (Reference Rognes1987) also reported synonyms under P. rudis that could not be confidently traced to the new species he was naming. In Hall’s (Reference Hall1948) monumental work on Calliphoridae (including Pollenia), only P. rudis was included. Later, Shewell (Reference Shewell1961) reported Pollenia vagabunda (Meigen, 1826) from Prince Edward Island, Nova Scotia, and British Columbia. Nevertheless, the practice seems to have been to assume that all Canadian Pollenia were rudis, including life history studies (e.g., Thomson Reference Thomson1972; Yahnke and George Reference Yahnke and George1972; Thomson and Davies Reference Thomson and Davies1973a, Reference Thomson and Davies1973b, Reference Thomson and Davies1974). However, Thomson and Davies (Reference Thomson and Davies1973b) did speculate that discrepancies in the literature with host interactions could be due to “various strains” of Pollenia.
Rognes (Reference Rognes1987) examined the specimens collected from the same area as those reported in Yahnke and George (Reference Yahnke and George1972) and reported both P. rudis and a new species, Pollenia pseudorudis (Rognes Reference Rognes1987), which is now regarded as a synonym of Pollenia pediculata Macquart, 1834. Rognes (Reference Rognes1987) noted that the majority of specimens used in Yahnke and George’s (Reference Yahnke and George1972) paper were of this latter species. Rognes (Reference Rognes1991) listed six species from North America in his revision of Scandinavian species.
Following this, Whitworth (Reference Whitworth2006) produced a key to the six species in North America, which was later used as the basis for a web-based illustrated key by Jewiss-Gaines et al. (Reference Jewiss-Gaines, Marshall and Whitworth2012). From specimens preserved in collections that could be re-evaluated for species identifications, Jewiss-Gaines et al. (Reference Jewiss-Gaines, Marshall and Whitworth2012) reported the earliest records as follows: 1904 – P. pediculata; 1906 – P. angustigena Wainwright, 1940; 1913 – P. rudis; 1925 – P. griseotomentosa (Jacentkovskỳ, 1944); 1958 – P. vagabunda; and 1969 – P. labialis. In terms of P. vagabunda, an earlier specimen from 1940 exists (Shewell Reference Shewell1961) and is housed at the Canadian National Collection of Insects, Arachnids, and Nematodes (Ottawa, Ontario, Canada). Recently, the subfamily Polleniinae of the Calliphoridae was elevated to family status, Polleniidae (Cerretti et al. Reference Cerretti, Stireman, Badano, Gisondi, Rognes, Lo Giudice and Pape2019).
Pollenia and earthworms
Most of what is known about the life history of Pollenia in North America was studied under the catch-all species name P. rudis. As a result, we use only the genus name for this section. Most studies on the biology of the immature stages of Pollenia were undertaken in Europe. The first record of Pollenia parasitising worms came from a German earthworm publication in 1845 by W.F.L. Hoffmeister (cited in Thomson Reference Thomson1972). In 1909, the information was repeated by Keilin (Reference Keilin1911; later republished in a North American journal in English in 1911). Pollenia larvae were not found in North America until 1916 (Webb and Hutchison Reference Webb and Hutchison1916), and although Keilin’s European research was built upon by North American researchers, the lumping of many species under the name of P. rudis makes it impossible to know which species the life history information applies to (Rognes Reference Rognes1987).
At minimum, at least one Pollenia species in North America is a parasitoid of earthworms (Oligochaeta: Lumbricidae) (Jewiss-Gaines et al. Reference Jewiss-Gaines, Marshall and Whitworth2012). Gravid females insert their ovipositors into the soil (DeCoursey Reference DeCoursey1927) and deposit eggs in batches of up to seven at a time until 100–130 eggs have been laid (Thomson and Davies Reference Thomson and Davies1973a). Individual batches are placed about 30 cm apart over a large area, which is an advantage to larvae seeking earthworms (DeCoursey Reference DeCoursey1927). Larvae search for earthworm hosts by an apparent random movement through naturally occurring pore spaces (Thomson and Davies Reference Thomson and Davies1973a), with most encounters taking place as earthworms move to the surface at night or after light rains (Heath et al. Reference Heath, Marris and Harris2004). Pollenia likely are free living in the soil when not feeding on earthworms (DeCoursey Reference DeCoursey1927). This behaviour, where eggs are laid away from a host and larvae navigate to their host unaided, is unusual for calyptrate Diptera (Wood Reference Wood1987).
Parasitised earthworms have been collected in North America and have also been successfully parasitised under controlled laboratory conditions (Yahnke and George Reference Yahnke and George1972; Thomson and Davies Reference Thomson and Davies1973b). Not all field-collected earthworm species were affected by larvae (Thomson and Davies Reference Thomson and Davies1973b), and earthworm species reported to be parasitised in Europe were not found to contain larvae in North America (Webb and Hutchison Reference Webb and Hutchison1916). Pollenia may also be considered predators, with larvae sometimes exiting the worm to feed from the outside (Szpila Reference Szpila2003).
Pollenia larvae have also been found to parasitise caterpillars and bees (van Emden Reference van Emden1954). Pollenia of an unknown species were reported feeding in honeybee (Hymenoptera: Apidae) thoraces in Egypt (Ibrahim Reference Ibrahim1984), and some species have been reared on noctuid moths (Lepidoptera: Noctuidea) (Rognes Reference Rognes2010). Larvae of Pollenia are obligate predators or parasites, however, and although they can feed on crushed fresh earthworms, they are unable to survive when fed on cow dung, horse manure, loam soil, clay soil, grass sod, decaying roots of grass in soil, decaying wood, decaying meat, or dead earthworms (DeCoursey Reference DeCoursey1927, but see van Emden (Reference van Emden1954) for one possible example where this was successful). The presence of Pollenia adults in buildings in areas far from suitable earthworm habitat suggests the need for further research into alternative hosts (Cranshaw and Due Reference Cranshaw and Due2018).
Life history and overwintering
In North America, there are three to four generations per year of Pollenia (Thomson and Davies Reference Thomson and Davies1973a), with populations peaking with the third overwintering generation in about early October. Adult males and females seek shelter without requiring either food or water (DeCoursey Reference DeCoursey1927). From these, flies that emerge early die in the snow or feed as outdoor temperatures permit (DeCoursey Reference DeCoursey1927). Females from P. pediculata and P. vagabunda overwinter as virgins with undeveloped ovaries until the spring (Greenberg Reference Greenberg1998), whereas males produce sperm continuously (DeCoursey Reference DeCoursey1927). After winter, when temperatures rise, adults copulate (Greenberg Reference Greenberg1998), with females ovulating about a month later (DeCoursey Reference DeCoursey1927). The overwintering generation dies off by about mid-April, and their offspring appear in late May or early June, with numbers remaining low until mid-July and then increasing until fall (DeCoursey Reference DeCoursey1927).
Pollenia and agriculture
Adults of this genus are reported to be significant pollinators (Būda et al. Reference Būda, Radžiutė and Lutovinovas2009; Jewiss-Gaines et al. Reference Jewiss-Gaines, Marshall and Whitworth2012), hence, the name Pollenia. There are reports of pollen-covered Pollenia (Robineau-Desvoidy Reference Robineau-Desvoidy1863), and adults are commonly found during general surveys of insects on a number of plants in North America, including wind-pollinated plants such as wheat (Poaceae) (Webster Reference Webster1900) and insect-pollinated plants such as carrot (Apiaceae) (Bohart and Nye Reference Bohart and Nye1960), ox-eye daisy (Asteraceae) (Judd Reference Judd1964), flowering boneset (Asteraceae) (Allan Reference Allan1967), and strawberry blossoms (Rosaceae) (Nye and Anderson Reference Nye and Anderson1974).
Although it is reasonable to assume that Pollenia’s nectar feeding results in pollination, neither the extent nor the importance of this is known. For example, in a comprehensive review of dipteran pollinators (Larson et al. Reference Larson, Kevan and Inouye2001), Pollenia, while listed in a table, were not mentioned in the discussion of important dipteran pollinators. The insects’ yellow hairs might give the appearance of carrying more pollen than is present. For example, during a field survey of insects associated with sugar beets (Amaranthaceae), individual Syrphidae (hover flies) were found to carry eight times as many pollen grains (11 619) as Pollenia (1421), which were also outclassed by Muscidae (1933) (Free et al. Reference Free, Williams, Longden and Johnson1975). Adult Pollenia also feed on the exudates of plants, carrion, faecal matter, and refuse (Thomson Reference Thomson1972) and on natural oils from yarns and spun goods (Mann Reference Mann1882).
Despite the potential importance to agriculture and wild ecosystems as both pollinators and parasitoids, little is known about the Pollenia species in North America, including their role in these ecosystems, their basic life history, and – the most fundamental question of all – where they are found. In this paper, we report on thousands of Pollenia collected from across Canada and update the known distributions based on our new records.
Materials and methods
All Pollenia specimens were collected as part of a large-scale collaboration with the Ontario Provincial Police and the Royal Canadian Mounted Police across Canada and are housed in the Entomology Lab at Trent University (Peterborough, Ontario, Canada). The initial study was designed to survey forensically important blow fly species (Diptera: Calliphoridae) of the subfamilies Calliphorinae, Luciliinae, and Chrysomyinae using baited bottle traps with the help of law-enforcement volunteers from across Canada. Details are provided in Langer et al. (Reference Langer, Kyle, Illes, Larkin and Beresford2019). Briefly, volunteers were invited from each province; 32 individuals responded from detachments in seven provinces: British Columbia, Alberta, Saskatchewan, Ontario, New Brunswick, Nova Scotia, and Newfoundland and Labrador. The unexpected abundance of Pollenia spp. that were also captured provided the impetus for this paper. Typically, bycatch specimens remain unanalysed, but when possible, reporting is encouraged, especially for nationwide surveys (Spears and Ramirez Reference Spears and Ramirez2015).
From 2011 to 2013, each volunteer was mailed two 2-L bottle traps baited with prerotted beef liver and four collecting bottles containing nontoxic plumbing antifreeze as a preservative. (See details of the bottle-trap design in Langer et al. Reference Langer, Kyle and Beresford2016.) After being deployed for two weeks at each location, the four bottles of captured specimens (one per week per trap) were mailed back to us for processing. When received, specimens were transferred to bottles with 80% ethanol until they could be pinned and identified. The identifications were confirmed by KAV; the key used is that in Jewiss-Gaines et al. (Reference Jewiss-Gaines, Marshall and Whitworth2012).
New provincial records and range extensions are based on existing known range records reported in Jewiss-Gaines et al. (Reference Jewiss-Gaines, Marshall and Whitworth2012) and Gisondi et al. (Reference Gisondi, Rognes, Badano, Pape and Cerretti2020) and on records in GBIF.org (Global Biodiversity Information Facility Secretariat 2021). Range maps shown here were created in ArcMap (Environmental Systems Research Institute 2011).
Results
We captured 2249 Pollenia specimens and identified 2211 of these to species level. Thirty-eight specimens were damaged and could not be identified. Pollenia were collected from 29 of the 32 locations surveyed and included all six species known from North America (Table 1). The most abundant species – and the only species found in all sampled provinces – was P. pediculata (n = 1272), followed by P. rudis (n = 435), P. angustigena (n = 175), P. labialis (n = 146), P. vagabunda (n = 138), and P. griseotomentosa (n = 45; Table 1).
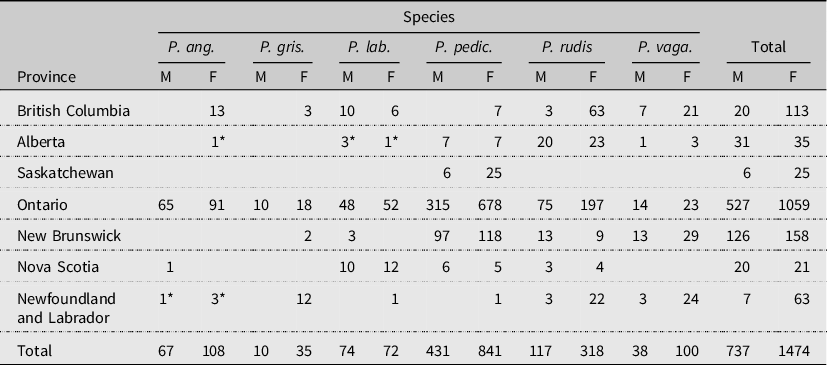
Table 1. Pollenia species collected in each province (west to east) from 2011 to 2013 by sex (M, male; F, female). An asterik (*) denotes a first provincial record. From left to right, P. angustigena, P. griseotomentosa, P. labialis, P. pediculata, P. rudis, and P. vagabunda.
Distribution
Despite the commonness of Pollenia and the low number of North American species, our collections resulted in three new provincial records: two from Alberta and one from Newfoundland and Labrador (Table 1; Figs. 1–6). The new record from Newfoundland and Labrador was a range extension, extending the known population further to the east (P. angustigena). Of the new records from Alberta, P. labialis served as a gap infill between records in the west, in British Columbia, and in the east, in Ontario.

Fig. 1. Distribution of P. angustigena collected during the present study. The map notes traps from which this species was collected (black circles) and absent (white circles). Map created in ArcMap.

Fig. 2. Distribution of P. griseotomentosa collected during the present study. The map notes traps from which this species was collected (black circles) and absent (white circles). Map created in ArcMap.
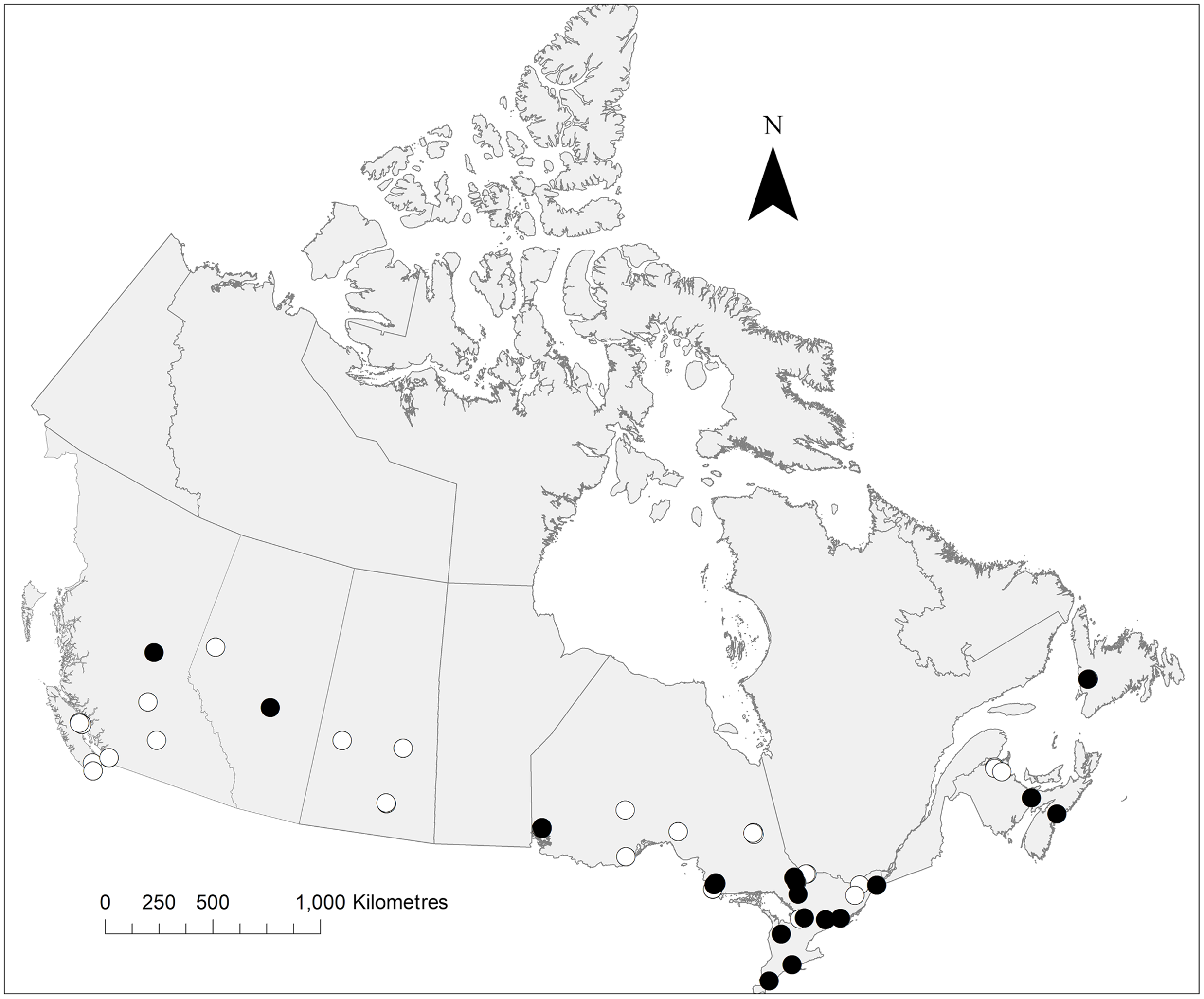
Fig. 3. Distribution of P. labialis collected during the present study. The map notes traps from which this species was collected (black circles) and absent (white circles). Map created in ArcMap.
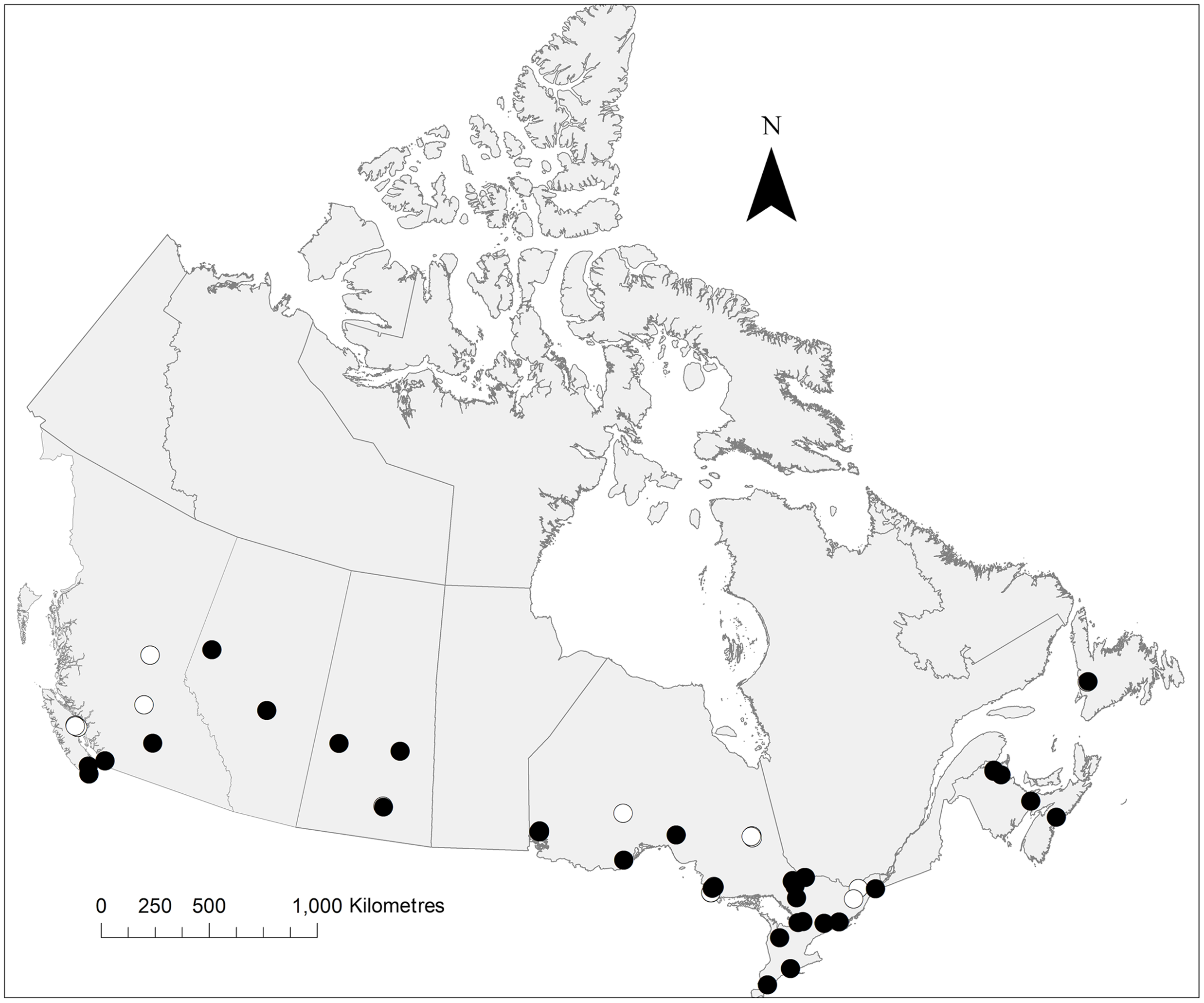
Fig. 4. Distribution of P. pediculata collected during the present study. The map notes traps from which this species was collected (black circles) and absent (white circles). Map created in ArcMap.

Fig. 5. Distribution of P. rudis collected during the present study. The map notes traps from which this species was collected (black circles) and absent (white circles). Map created in ArcMap.

Fig. 6. Distribution of P. vagabunda collected during the present study. The map notes traps from which this species was collected (black circles) and absent (white circles). Map created in ArcMap.
The known distributions of all species, with the exception of P. pediculata and P. rudis, are based largely on pockets of records from the west coast, from the east coast, and in southern Ontario and Québec. Comparatively few records are reported from Alberta, Saskatchewan, Manitoba, and western Ontario (Jewiss-Gaines et al. Reference Jewiss-Gaines, Marshall and Whitworth2012). This holds true when regarding North American Pollenia populations as a whole: although their populations extend south into the United States of America, large gaps remain towards these middling latitudes (Jewiss-Gaines et al. Reference Jewiss-Gaines, Marshall and Whitworth2012). Our records fill some of these gaps for P. angustigena (Alberta), P. labialis (Alberta and western Ontario), and P. rudis (western Ontario).
We caught more Pollenia in the first of the two sampling weeks (week 1: 1270; week 2: 941). Collection bottles were changed between weeks, but the liver bait was not refreshed. We also caught more females than males (females: 1474; males: 737), nearly twice as many each week (week 1 – females: 854, males: 416; week 2 – females: 620, males: 321). By species, we caught more females for all species except Pollenia labialis, for which two more males than females were caught (Table 1).
Across all sampling sessions and locations, 37% of traps contained one or more Pollenia specimens, with the mean being 11.46 specimens per trap. The most caught in any one trap was 195 specimens, from Peterborough, Ontario. Although per-trap catches were low in most cases, Pollenia were a consistent bycatch of our liver-baited bottle traps. Approximately three times fewer Pollenia were collected than blow flies – the original target of these traps: 2211 Pollenia versus 7272 blow flies (Langer et al. Reference Langer, Kyle, Illes, Larkin and Beresford2019; 0.304), which is far more Pollenia than we had expected from traps designed for carrion specialists.
All six species were caught in British Columbia, Newfoundland and Labrador, and Ontario, with 150 specimens or less each (Table 1). Provinces with the fewest species were Nova Scotia and Saskatchewan. Even with 284 specimens, only five species were captured in New Brunswick. Regionally, the Prairie provinces have the fewest species of Pollenia.
Discussion
Although this genus is commonly encountered in general surveys, we were able to report a few first provincial records (Table 1). This likely reflects the relatively little attention that has been paid to the genus. For example, our records expand the known range of two of the six North American species, which is unprecedented for such large-bodied insects with potential importance in agricultural systems and as pests. There are very few records from Manitoba and Saskatchewan. Pollenia are present in Manitoba, listed in pest prevention reports for the province as cluster flies (Ellis Reference Ellis2002), with records for P. pediculata and P. rudis available online (Global Biodiversity Information Facility Secretariat 2021). However, the species present in Manitoba have yet to be published. The situation in Saskatchewan is similar: we recorded P. pediculata, which was previously recorded from Saskatchewan in 2020 (Gisondi et al. Reference Gisondi, Rognes, Badano, Pape and Cerretti2020). The dearth of records from the Canadian prairies is likely due to a combination of fewer collections and of fewer Pollenia species being present.
Introduction and spread
If Pollenia were brought to the Nearctic through shipping, their earliest occurrences would have been near port and coastal cities (British Columbia coast, Atlantic coast, and Great Lakes region), with Pollenia dispersing inland towards land-locked regions (Alberta, Manitoba, and Saskatchewan). Although this account of the genus’s spread is speculative, it appears to be consistent with what we see from their current distribution maps. Dall’s (Reference Dall1882) account of Pollenia appearing in Geneva, New York in about the 1850s is especially interesting because it is not far from Oswego, in the same state, home of one of the busiest shipping ports on the Great Lakes during the 1850s (Palmer Reference Palmer2010). Howard (Reference Howard1911) believed that Pollenia may have first arrived on ships while hibernating, a reasonable assertion because Pollenia have successfully crossed oceans on cargo ships. For example, in 1981, they were intercepted in New Zealand on cargo ships from the United States of America (Dear Reference Dear1986). The spread of Pollenia in North America continues even now, with P. vagabunda recently reported in Alaska (Bowser Reference Bowser2015).
Because Pollenia are earthworm parasitoids, earthworm hosts likely must be common in an area for Pollenia to exist, although which species are parasitoids is not yet known. Currently, only four species of earthworms known to host Pollenia exist in Canada and are found in all provinces: Allolobophora chlorotica (Savigny, 1826) (Crassiclitellata: Lumbricidae), Aporrectodea rosea (Savigny, 1826) (Crassiclitellata: Lumbricidae), Aporrectodea trapezoides (Duges, 1828) (Crassiclitellata: Lumbricidae), and Lumbricus terrestris Linnaeus, 1758 (Opisthopora: Lumbricidae) (Tomlin and Fox Reference Tomlin and Fox2003; Reynolds Reference Reynolds2021).
Sex ratio
We captured more females with the carrion-baited bottle traps. Estimates of Pollenia sex ratios from overwintering flies suggest that populations of P. pediculata and P. rudis have more males and that P. angustigena and P. vagabunda have more females (Greenberg Reference Greenberg1998). In New Zealand, sex ratios in overwintering flies are closer to 50:50 (Heath et al. Reference Heath, Marris and Harris2004). This might reflect overwintering behaviour: females tend to overwinter in outdoor refugia such as corn stubble, whereas males tend to overwinter in buildings (DeCoursey Reference DeCoursey1927), but this is not conclusive. Summer sex ratio records are few: Hall (Reference Hall1948) reported mostly males found on wild parsnip. Our results likely represent a trapping bias of our methods.
Sampling bias
Although blow flies were the original target for our liver-baited traps, they captured a surprising number of Pollenia. Pollenia are not unusual in carrion-baited traps (Feddern et al. Reference Feddern, Amendt, Schyma, Jackowski and Tschui2018), but the reasoning for this is not yet known (Baz et al. Reference Baz, Cifrián, Díaz-äranda and Martín-Vega2007). Pollenia spp. have little to no forensic importance (Greenberg Reference Greenberg1998; Brundage et al. Reference Brundage, Bros and Honda2011; Feddern et al. Reference Feddern, Amendt, Schyma, Jackowski and Tschui2018). Pollenia have been captured at whole carcasses (Tabour et al. Reference Tabour, Fell and Brewster2005; Bugajski et al. Reference Bugajski, Seddon and Williams2011; Benbow et al. Reference Benbow, Lewis, Tomberlin and Pechal2013; Šuláková and Barták Reference Šuláková and Barták2013; Weidner et al. Reference Weidner, Gemmellaro, Tomberlin and Hamilton2017), leading to suggestions that earthworms near the carrion could be the attracting source (Šuláková and Barták Reference Šuláková and Barták2013). However, Pollenia do not deposit eggs on earthworms but on soil, making this explanation unlikely. Pollenia have also been collected in baited bottle traps that use beef liver (Brundage et al. Reference Brundage, Bros and Honda2011; Weidner et al. Reference Weidner, Jennings, Tomberlin and Hamilton2015; Feddern et al. Reference Feddern, Amendt, Schyma, Jackowski and Tschui2018) and pig liver (Hwang and Turner Reference Hwang and Turner2005; Farinha et al. Reference Farinha, Dourado, Centeio, Oliveira, Dias and Rebelo2014). Weidner et al. (Reference Weidner, Gemmellaro, Tomberlin and Hamilton2017) suggested that some unknown specific chemical released by beef liver but not unique to it could attract species that do not use the bait for colonisation.
Other successful baits for capturing Pollenia include bananas (Webb and Hutchison Reference Webb and Hutchison1916; Yahnke and George Reference Yahnke and George1972), especially when combined with milk and vanilla extract (Hall Reference Hall1948), and apples (DeCoursey Reference DeCoursey1927), consistent with the need to feed from flowering plants, rotten fruits, and souring tree sap (Hall Reference Hall1948). Only two plant compounds are known to attract Pollenia; these are methyl eugenol and methyl salicylate (Būda et al. Reference Būda, Radžiutė and Lutovinovas2009; El-Sayed Reference El-Sayed2021). Methyl eugenol is released when damage occurs to the leaves, stems, roots, fruits, or flowers of more than 450 plant species and can deter animals from feeding on plant tissues (Tan and Nishida Reference Tan and Nishida2012). Methyl salicylate is also released as a response to plant damage and is attractive to many predator insects that prey upon insect herbivores (Stepanycheva et al. Reference Stepanycheva, Petova, Chermenskaya and Shamshev2016). Methyl salicylate is emitted by many flowers and could signal a nectar source for Pollenia, which would allow the plant to be pollinated (Būda et al. Reference Būda, Radžiutė and Lutovinovas2009). None of these compounds are known to attract members of Calliphoridae (El-Sayed Reference El-Sayed2021).
Our captures of Pollenia might be due to methyl salicylate in the propylene glycol antifreeze that was used as a trap preservative (Thomas Reference Thomas2008). Methyl salicylate is added as a scent so that antifreeze can be detected in plumbing (Cook Reference Cook1998). Propylene glycol is a nontoxic alternative to ethylene glycol (Skvarla et al. Reference Skvarla, Larson and Dowling2014) and is an effective preservative for a range of insect studies (Weigand et al. Reference Weigand, Desquiotz, Weigand and Szucsich2021). We were unable to determine if methyl salicylate was added to the brand of plumbing antifreeze that we used. However, this compound does not explain Pollenia’s prevalence in other studies that did not use plumbing antifreeze in their traps (e.g., Hwang and Turner Reference Hwang and Turner2005; Brundage et al. Reference Brundage, Bros and Honda2011; Farinha et al. Reference Farinha, Dourado, Centeio, Oliveira, Dias and Rebelo2014; Fremdt and Amendt Reference Fremdt and Amendt2014; Weidner et al. Reference Weidner, Jennings, Tomberlin and Hamilton2015; Feddern et al. Reference Feddern, Amendt, Schyma, Jackowski and Tschui2018) and why fewer Pollenia are captured when whole animals are used as carrion bait.
Knowledge gaps
Because most of our understanding of the natural history of Pollenia comes from research that lumped several species as P. rudis (e.g., Thomson Reference Thomson1972; Yahnke and George Reference Yahnke and George1972; Thomson and Davies Reference Thomson and Davies1973a, Reference Thomson and Davies1973b, Reference Thomson and Davies1974), the basic natural history needs to be re-examined for each species (Rognes Reference Rognes1987). Our knowledge gaps include such basic information as (1) which North American Pollenia species are earthworm parasitoids; (2) how important this is for each species; (3) what other earthworms can host immature Pollenia; (4) whether Pollenia species differ in terms of earthworm host species; (5) how important Pollenia are for earthworm population dynamics and mortality; (6) whether Pollenia exist outside of regions with earthworms; (7) how important Pollenia species are for pollination services in agriculture; (8) how important Pollenia species are for transporting pathogens; and (9) what species of Pollenia exist across Canada.
Conclusion
We hope that this work spurs interest in this genus and leads to future studies aimed at better understanding the distribution and biology of Pollenia species. Although our work summarises what we know of their biology in North America and fills gaps in their distribution in Canada, much more can be done. The gaps in our understanding are curious and perhaps reflect the common state of entomological and biodiversity studies in general – that there is much that we do not know and much that we take for granted.
Acknowledgements
This work would not have been possible without the many volunteers from the Ontario Provincial Police and the Royal Canadian Mounted Police who deployed and collected our traps and specimens. The authors thank Mike Illes, Brian Yamashita, and Christopher Kyle, who made many of the present study’s volunteer collaborations possible. The authors also thank Scott Larkin and Donald Bourne for their work on constructing and mailing traps, Jesyka Galasso, Adam Bear, and Giselle Bezanson for volunteering time to help prepare samples, and Mohammed Samkari, whose support was invaluable. Funding was provided by the Canadian Police Research Centre, CPRC Research Project (Grant No. 91067), the ENLS graduate studies program, Trent University, and the Saudi Arabian Cultural Bureau. Two anonymous reviewers and Bradley Sinclair kindly commented on earlier drafts of this paper.
Competing interests
The authors declare they have no competing interests.










