Introduction
Swede midge, Contarinia nasturtii (Kieffer) (Diptera: Cecidomyiidae), a galling fly, is a challenging invasive pest of Brassica Linnaeus spp. (Brassicaceae) crops in North America. Since its introduction to Ontario, Canada from Europe in the 1990s (Hallett and Heal Reference Hallett and Heal2001), swede midge has spread to several provinces within Canada and states within the United States of America. The midge has caused economic losses of canola (Brassica napus Linnaeus), broccoli and cabbage (Brassica oleracea Linnaeus), and other related crops in Ontario and Québec, Canada and in New York and Vermont, United States of America (Hallett and Heal Reference Hallett and Heal2001; Chen et al. Reference Chen, Shelton, Hallett, Hoepting, Kikkert and Wang2011). Climatic models predict that swede midge could establish in nearly all vegetable-producing areas in the eastern United States of America and in canola-producing provinces of the Canadian Prairies (Mika et al. Reference Mika, Weiss, Olfert, Hallett and Newman2008), threatening the economic viability of Brassica vegetable and oilseed production in North America. Each year, the midge is identified in new states and provinces. Due to challenges associated with identifying and managing swede midge, populations have grown to devastating levels on individual farms, with up to 100% crop loss reported (Hallett and Heal Reference Hallett and Heal2001; Chen et al. Reference Chen, Shelton, Hallett, Hoepting, Kikkert and Wang2011).
As a cecidomyiid fly, swede midge has the ability to manipulate plant growth (Gagné Reference Gagné1989), leading to distorted and unmarketable crops. Digestive secretions by cecidomyiid larvae break down plant cells and alter plant nutrient allocation and hormone dynamics within the plant (Tooker and De Moraes Reference Tooker and De Moraes2007, Reference Tooker and De Moraes2010). Adult swede midge oviposit onto the meristems of host plants, where larvae feed within the meristematic tissue (Gagné Reference Gagné1989). Larval feeding causes scarred and deformed growth, rendering leaves, stems, and heads unmarketable (Readshaw Reference Readshaw1961; Hallett Reference Hallett2007; Chen et al. Reference Chen, Shelton, Hallett, Hoepting, Kikkert and Wang2011; Stratton et al. Reference Stratton, Hodgdon, Zuckerman, Shelton and Chen2018). Heading Brassica vegetables are particularly sensitive to larval feeding. A single larva can render a cauliflower plant unmarketable, and plants are susceptible to damage from the seedling to heading stages (Stratton et al. Reference Stratton, Hodgdon, Zuckerman, Shelton and Chen2018). Because damage symptoms are often visible only after larvae vacate the plant to pupate within the soil (Stratton et al. Reference Stratton, Hodgdon, Zuckerman, Shelton and Chen2018), growers often mistake midge feeding damage for nutrient deficiencies (Hallett and Heal Reference Hallett and Heal2001). Misdiagnosis of midge damage allows populations to build unchecked.
The cryptic feeding behaviour of the larvae poses additional challenges for the use of insecticides for swede midge management. Because larvae are protected within young leaves in the meristem, insecticides without systemic action are seldom reliable due to poor contact with the feeding insects (Hallett et al. Reference Hallett, Chen, Sears and Shelton2009a; Seaman et al. Reference Seaman, Lange and Shelton2013; Evans and Hallett Reference Evans and Hallett2016). Current recommendations for conventional swede midge management include calendar applications of insecticides, including neonicotinoids, pyrethroids, and spirotetremat (Hallett et al. Reference Hallett, Chen, Sears and Shelton2009a; Chen and Shelton Reference Chen and Shelton2010). Systemic neonicotinoids are particularly effective against the midge. The recommendation for calendar sprays of insecticides, however, negates decades of integrated pest management recommendations to reduce reliance on chemical control (Andaloro et al. Reference Andaloro, Hoy, Rose and Shelton1983; Chen et al. Reference Chen, Shelton, Hallett, Hoepting, Kikkert and Wang2011). If governmental agencies ban neonicotinoids in the future, conventional and organic growers alike may be searching for nonchemical tactics for swede midge management. Unfortunately, none of the pesticides currently allowed in organic production is consistently effective for swede midge management, particularly at high population densities (Seaman et al. Reference Seaman, Lange and Shelton2013; Evans and Hallett Reference Evans and Hallett2016). As a result, growers managing their crops organically must rely on alternatives to insecticides and ecologically based management strategies, which often fall short in protecting crops (Hodgdon et al. Reference Hodgdon, Hoepting, Hallett and Chen2017).
Several commonly used biological, cultural, and physical control tactics are not effective in managing swede midge. Aspects of the midge life cycle and ecology pose challenges to management. With more than one emergence phenotype and multiple overlapping generations (Hallett Reference Hallett2007; Hallett et al. Reference Hallett, Goodfellow, Weiss and Olfert2009b; Des Marteaux et al. Reference Des Marteaux, Schmidt, Habash and Hallett2015), swede midge is present throughout the growing season in North America. Therefore, management strategies must provide season-long protection. Surveys in Europe for natural enemies rendered no suitable candidates for biological control programmes (Corlay et al. Reference Corlay, Boivin and Bélair2007; Abram et al. Reference Abram, Haye, Mason, Cappuccino, Boivin and Kuhlmann2012). Although management strategies such as insect exclusion netting and crop rotation can be effective as alternatives to insecticides for swede midge management (Chen et al. Reference Chen, Shelton, Hallett, Hoepting, Kikkert and Wang2011; Hodgdon et al. Reference Hodgdon, Hoepting, Hallett and Chen2017; Hoepting and Vande Brake Reference Hoepting and Vande Brake2020), these options often present logistic and economic challenges for growers.
Because damage thresholds for swede midge larvae in Brassicas are so low and larvae are difficult to control (Stratton et al. Reference Stratton, Hodgdon, Zuckerman, Shelton and Chen2018), management strategies that prevent oviposition are urgently needed. Although “scout and spray” pest management algorithms are effective for other pests of Brassica crops, such as lepidopteran pests with highly visible larval stages and higher damage thresholds (Andaloro et al. Reference Andaloro, Hoy, Rose and Shelton1983), preventing swede midge damage appears to be more complex. Field scouting for larvae is impractical because larvae are hidden within the meristem. Adult midges are difficult to identify in traps: one must use molecular identification to distinguish lookalike species. In addition, preventing oviposition and larval feeding within the meristem rather than relying on curative measures to kill hidden larvae may be necessary to reduce crop damage from this devastating pest.
Pheromone-mediated mating disruption is a pest management strategy whereby large quantities of synthetic female sex pheromone are applied to crops to disorient males and prevent mating. Pheromone-mediated mating disruption is promising for swede midge management because it prevents mating and thereby indirectly prevents oviposition. Although this tactic has been successful for managing lepidopteran pests in perennial orchard and vineyard systems, decreasing insecticide use and limiting impacts on nontarget organisms (Welter et al. Reference Welter, Pickel, Millar, Cave, Van Steenwyk and Dunley2008; Witzgall et al. Reference Witzgall, Kirsch and Cork2010), it has not been widely implemented for nonlepidopteran pests and pests of annual crops (Miller and Gut Reference Miller and Gut2015). Cost and migrating gravid females are major challenges associated with using mating disruption in annual crops (Fadamiro et al. Reference Fadamiro, Cossé and Baker1999; Welter et al. Reference Welter, Pickel, Millar, Cave, Van Steenwyk and Dunley2008; Vacas et al. Reference Vacas, Alfaro, Primo and Navarro-Llopis2011).
Although Samietz et al. (Reference Samietz, Baur and Hillbur2012) demonstrated that pheromone-mediated mating disruption was effective for swede midge management in a European study, considerable economic challenges must be addressed for commercial adoption. The female swede midge sex pheromone, a 1:2:0.02 mixture of (2S,9S)-diacetoxyundecane, (2S,10S)-diacetoxyundecane, and (S)-2-acetoxyundecane (Hillbur et al. Reference Hillbur, Celander, Baur, Rauscher, Haftmann, Franke and Francke2005), is costly to synthesise due to the presence of either one or two chiral centres in each component (Samietz et al. Reference Samietz, Baur and Hillbur2012). Therefore, multiple stereoisomers (three-dimensional configurations) are possible for each compound. Racemic pheromone compounds, or mixtures of all possible stereoisomers of the pheromone compounds, are typically less costly to produce and may present a more economical mating disruption system for swede midge. Although unnatural stereoisomers of the “main” (most abundant) swede midge pheromone component in the blend, (2S,10S)-diacetoxyundecane, inhibit male attraction (Boddum et al. Reference Boddum, Skals, Wirén, Baur, Rauscher and Hillbur2009; Hodgdon et al. Reference Hodgdon, Hallett, Stratton and Chen2019a), they may still be useful for mating disruption. Unattractive compounds may camouflage calling females, adulterate attractive pheromone plumes, desensitise males, and otherwise prevent males from locating females (Miller et al. Reference Miller, Gut, de Lame and Stelinski2006; Miller and Gut Reference Miller and Gut2015). We previously found that racemic pheromone blends prevented male midges from mating with calling females in a mating disruption simulation trial in the laboratory (Hodgdon et al. Reference Hodgdon, Hallett, Stratton and Chen2019a).
Unattractive and single-component racemic pheromone blends have been used successfully in pheromone-mediated mating disruption systems (Mafi et al. Reference Mafi, Vang, Nakata, Ohbayashi, Yamamoto and Ando2005; Higbee and Burks Reference Higbee and Burks2008; Onufrieva et al. Reference Onufrieva, Thorpe, Hickman, Leonard, Mastro and Roberts2008; Arakaki et al. Reference Arakaki, Hokama, Nagayama, Yasui, Fujiwara-Tsujii and Tanaka2013). In addition to using three-component racemic blends for swede midge, deploying the most attractive compound alone in its natural (2S, 10S)-diacetoxyundecane or racemic form may present another opportunity for lowering the cost of a mating disruption system. Unexpectedly, male midges possess antennal receptors for at least one of the unnatural stereoisomers within the racemic blend of 2,10-diacetoxyundecane (Boddum et al. Reference Boddum, Skals, Hill, Hansson and Hillbur2010). Racemic and single-component pheromone blends have yet to be tested for swede midge mating disruption.
Here, we assessed the efficacy of stereospecific, racemic, and single-component pheromone-mediated mating disruption treatments in small field plots of broccoli (Brassica oleracea Linnaeus var. italica). Using reservoir-type mating disruption dispensers, we tested whether male trap counts (trap shutdown) and crop damage differed between plots treated with three-component, single-component, stereospecific, or racemic pheromone blends. We use our results to identify the most promising candidate pheromone blend for swede midge mating disruption. Lastly, we discuss future research directions to address ecological challenges associated with implementing pheromone-mediated mating disruption in complex annual cropping systems.
Materials and methods
Experimental sites
We tested the mating disruption treatments at a total of three field sites in Ontario and Québec, Canada, over two field seasons. Swede midge was first documented in North America in Ontario in 2000 (Hallett and Heal Reference Hallett and Heal2001); therefore, populations are well established in this region. For the three-component pheromone experiment, our test plots were located at the University of Guelph Elora Research Station (“Elora”), Ontario and at a large commercial vegetable farm in New Hamburg (“New Hamburg”), Ontario in 2016 and 2017. For the single-component experiment conducted in 2017 and 2018, we used three field sites: Elora, New Hamburg, and a third site at the Institut de recherche et de développement en agroenvironnement (IRDA) in Saint-Bruno-de-Montarville (“Saint-Bruno”), Québec, Canada. The New Hamburg and Saint-Bruno sites were certified organic.
To ensure swede midge pressure, we situated the experimental plots at each location in close proximity to, but not within, fields previously cropped in the last year with Brassica oilseed or vegetable crops. At the Elora site, wheat (Triticum aestivum Linnaeus) (Poaceae), corn (Zea mays Linnaeus) (Poaceae), canola (Brassica napus Linnaeus), and soya bean (Glycine max (Linnaeus) Merrill) (Fabaceae) comprised the major components of the surrounding cropping systems. Mixed vegetable crops (including Brassica and Raphinus spp.) (Brassicaceae) were grown at the New Hamburg site. Mixed vegetable crops and grassland comprised a majority of the landscape at the Saint-Bruno site.
We used randomised complete block designs to test three pheromone treatments: stereospecific (natural), racemic, and a nontreated control. We used 16 × 16-m plots of broccoli, based on experimental designs used by Samietz et al. (Reference Samietz, Baur and Hillbur2012). We tested three- and single-component treatments in separate experiments. Each block contained one plot of each treatment (three plots per block), with a total of six three-component treatment replicates (one block at Elora and two blocks at Saint-Bruno in each of 2016 and 2017) and four single-component treatment replicates (one block at each site in 2017 (three blocks) and one block at Elora in 2018) per experiment (Table 1). To avoid the spread of pheromone plumes from one plot to another, we separated plots by a minimum of 425 m, similar to Samietz et al. (Reference Samietz, Baur and Hillbur2012). We situated each of the three plots per block as equidistant as possible from known infested fields to ensure equal swede midge pressure.
Table 1. Plot and broccoli production characteristics at experimental sites.
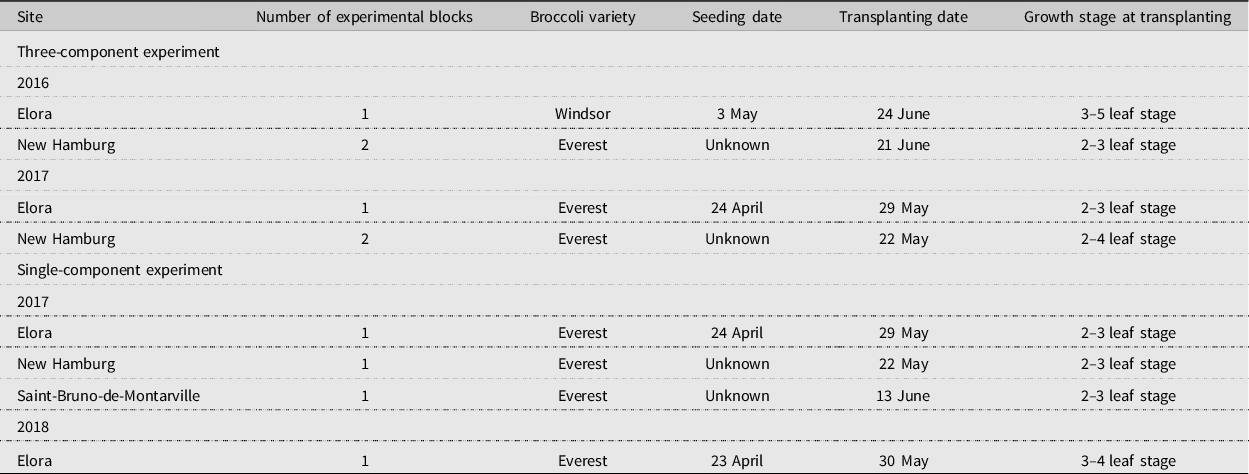
Each plot consisted of 20 rows of broccoli, using 30-cm within-row and 76-cm between-row spacing (∼1,040 plants per plot). We used ‘Everest’ broccoli, with the exception of ‘Windsor’ (Stokes Seed Ltd., Thorold, Ontario) planted at Elora in 2016. Both varieties are marketed for late-season crops and medium crown size and were selected by the commercial grower in New Hamburg for their crown size and market suitability. The two varieties do not differ in susceptibility to swede midge (Hallett Reference Hallett2007). We seeded broccoli a minimum of five weeks before transplanting, which occurred in May or June (Table 1), corresponding with the time of year when overwintering midges begin to emerge from the soil in Ontario (Hallett et al. Reference Hallett, Goodfellow, Weiss and Olfert2009b). We grew seedlings with either conventional (Elora) or certified organic (New Hamburg and Saint-Bruno) peat-based potting media and fertilisers in heated greenhouses, and then we transplanted the plants when they had 2–5 true leaves. We irrigated plots by hand immediately following transplanting. Throughout the remainder of the experiment, the plots received only natural rainfall, with the exception of the Saint-Bruno site, which received drip irrigation throughout the season. Broccoli production practices, including fertilisation and weed management, followed typical regimes for Ontario and Québec (Loughton Reference Loughton2013). We hand-weeded the plots until head formation and did not use pesticides.
Mating disruption treatments
We used reservoir-type pheromone dispenser bags to test the pheromone treatments (ChemTica Internacional, S.A., Heredia, Costa Rica), using previously tested spacing and release rates (Samietz et al. Reference Samietz, Baur and Hillbur2012). Each dispenser consisted of a brown polyethylene bag that contained the pure pheromone held within a microcentrifuge tube with a small hole to release the pheromone. The bag protected the pheromone from ultraviolet light and enabled a slow release of the pheromone through the semipermeable material. Each dispenser contained 100 times the pheromone amount used for monitoring (Samietz et al. Reference Samietz, Baur and Hillbur2012). In order to hold the biologically active stereoisomer ((S,S)- and (S)-) quantities constant across the pheromone treatments as recommended by Samietz et al. (Reference Samietz, Baur and Hillbur2012), we quadrupled or doubled each component for the racemic treatments (Table 2). We hung the dispenser bags approximately 25 cm above the ground on hooked wire flag posts arranged in the field in a staggered 2 × 2-m grid pattern, with a total of 80 bags per plot. We set up the dispensers the same day that the broccoli was transplanted. No bags or posts were set up within the control plots. Bags were installed once and were not replaced during the season.
Table 2. Amounts of pheromone components per reservoir dispenser in three- and single-component experiments. No dispensers were installed in control (nontreated) plots.
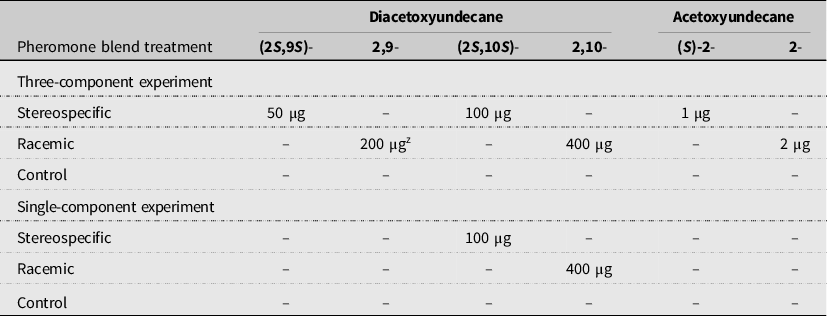
z Racemic pheromone blends were either quadrupled or doubled to deliver equal amounts of the biologically active (S,S)- or (S)-stereoisomers, respectively. The (S,S)-stereoisomers comprise one-quarter of the (2,9)-diacetoxyundecane and (2,10)-diacetoxyundecane racemic blends, whereas the 2-acetoxyundecane racemic blend consists of one-half (S)-stereoisomer (Hillbur et al. Reference Hillbur, Celander, Baur, Rauscher, Haftmann, Franke and Francke2005).
Evaluation of mating disruption
We used trap shutdown to assess whether the pheromone treatments disrupted the males’ ability to locate monitoring traps baited with the swede midge pheromone lures, a proxy for calling females. When mating disruption treatments are effective, few to no males are caught in sticky traps baited with commercial pheromone lures (Howse et al. Reference Howse, Stevens and Jones1998). One week after transplanting and dispenser deployment, we installed four traps per plot in random locations at least 5 m from the edges of the plots and apart from each other. Each trap consisted of a white delta (“Jackson”) trap body with a sticky card liner (Solida Distributions, Saint-Ferréol-les-Neiges, Québec) and polyethylene cap commercial lure containing 500 ng (2S,9S)-diacetoxyundecane, 1 μg (2S,10S)-diacetoxyundecane, and 10 ng (S)-2-acetoxyundecane (PheroNet Swede Midge Lures, Andermatt Biocontrol, Grossdietwil, Switzerland). Traps hung from wooden stakes 25 cm above the soil surface within the crop canopy. Lures were replaced twice during the experiment, at 30 and 60 days after transplanting. We counted males caught on the sticky cards in the traps weekly for 12 weeks. For trap counts and other data, we used a plot-level unit of measurement, calculating mean measurements from our subsamples for each plot.
We evaluated both broccoli plant damage and yield to assess mating disruption efficacy. We evaluated plants for swede midge damage to vegetative plant parts at three and six weeks after transplanting, using a four-point damage scale of increasing damage severity (Hallett Reference Hallett2007), where “0” equalled no swede midge damage, “1” equalled minor swelling, scarring, or deformation of meristem, petioles, and leaves, “2” equalled moderate to severe swelling, scarring, or deformation of meristem, petioles, and leaves, and “3” equalled complete death of apical meristem. Using a random number generator, we selected three locations per row of broccoli and scored three plants per row, for a total of 60 plants per plot. At the end of the season, we obtained a yield estimate of the broccoli in each plot. We evaluated swede midge damage to broccoli crowns using a six-point damage scale when broccoli heads were ready for harvest, between nine and 12 weeks after transplanting. Plants with no damage received a score of “0”. Plants with petiole scarring, head unevenness, or accompanying deformity due to larval feeding received scores from “1” to “4” with increasing severity, with a score of “5” given to plants in which the apical meristem died completely. We then used a separate binary scoring system to further categorise heads as either marketable or unmarketable, counting heads receiving a damage score of “0” as marketable, and from “1” to “5” as unmarketable, based on commercial quality standards for insect damage (United States Department of Agriculture 2006).
Statistical analyses
To test for differences in numbers of males trapped in plots over time, we used the generalised estimating equations extension for generalised linear models using SPSS, version 24 (IBM, Armonk, New York, United States of America). We used these extensions because they are robust to nonnormally distributed count data and allow for repeated measures (Zeger et al. Reference Zeger, Liang and Albert1988; Muff et al. Reference Muff, Held and Keller2016). We analysed pooled data across years and for each year separately, which allowed us to test both overall (pooled) and individual yearly results. We specified “plot” as subjects in the generalised estimating equation menu. Trap counts followed Poisson distributions, and pheromone and block were included as variables in all models. When we pooled data across years, we included year and year × pheromone variables.
To test whether broccoli damage differed between mating disruption plots and nontreated controls, we used ordinal logistic regression using the predictor variables described above. We also tested whether our counts of marketable broccoli crowns (yield) differed across treatments. Because our yield data consisted of nonnormally distributed ranks, we used nonparametric Kruskal–Wallis tests with pairwise post hoc Mann–Whitney U comparison tests with a Bonferroni correction. For all models, we evaluated significance using α = 0.05.
Results
Trap shutdown
We captured significantly fewer males in monitoring traps in the three-component stereospecific- and racemic pheromone-treated plots compared with the control (pooled:
![]() ${{\chi}_{2}}^{{2}}$
= 79.30, P < 0.001). In 2017, the pairwise comparisons indicated that male trap counts significantly differed between the stereospecific and racemic plots; however, they did not significantly differ in 2016 (Fig. 1). The mean numbers of males caught in traps were 4.8 ± 1.4 and 11.3 ± 4.0 per week for the stereospecific and racemic treatments, respectively, compared with 86.8 ± 15.7 males in the control plots across both years. These trap counts represent 95% (stereospecific) and 87% (racemic) reductions in trap counts compared with the nontreated control.
${{\chi}_{2}}^{{2}}$
= 79.30, P < 0.001). In 2017, the pairwise comparisons indicated that male trap counts significantly differed between the stereospecific and racemic plots; however, they did not significantly differ in 2016 (Fig. 1). The mean numbers of males caught in traps were 4.8 ± 1.4 and 11.3 ± 4.0 per week for the stereospecific and racemic treatments, respectively, compared with 86.8 ± 15.7 males in the control plots across both years. These trap counts represent 95% (stereospecific) and 87% (racemic) reductions in trap counts compared with the nontreated control.

Fig. 1. Mean numbers of males (± standard errors of the mean) caught in monitoring traps in three-component mating disruption plots each week. *** indicates statistical significance of the overall model with pheromone as a predictor of trap counts (2016:
![]() ${{\chi}_{2}}^{{2}}$
= 33.079, P < 0.001; 2017:
${{\chi}_{2}}^{{2}}$
= 33.079, P < 0.001; 2017:
![]() ${{\chi}_{2}}^{{2}}$
= 103.384, P < 0.001). Treatment lines marked with different letters are significantly different based on post hoc pairwise comparison tests (P < 0.05).
${{\chi}_{2}}^{{2}}$
= 103.384, P < 0.001). Treatment lines marked with different letters are significantly different based on post hoc pairwise comparison tests (P < 0.05).
Unlike the three-component study, we did not observe trap shutdown in the single-component experiment (Fig. 2). The pheromone treatments did not influence weekly male trap counts (pooled:
![]() ${{\chi}_{2}}^{{2}}$
= 1.373, P > 0.05). Overall, the trap counts were lower in our single-component experiment compared to the three-component experiment. At one site (Saint-Bruno), swede midge populations were very low (< 1 male per trap per day) for several weeks at the start of the experiment, then they increased in the final weeks of the experiment, allowing us to test for trap shutdown.
${{\chi}_{2}}^{{2}}$
= 1.373, P > 0.05). Overall, the trap counts were lower in our single-component experiment compared to the three-component experiment. At one site (Saint-Bruno), swede midge populations were very low (< 1 male per trap per day) for several weeks at the start of the experiment, then they increased in the final weeks of the experiment, allowing us to test for trap shutdown.

Fig. 2. Mean numbers of males (± standard errors of the mean) caught in monitoring traps in single-component mating disruption experiment plots each week. The lack of error bars for 2018 is due to the presence of only one replicate in this year. NS indicates nonsignificant differences in trap counts across pheromone treatments in 2017 (
![]() ${{\chi}_{2}}^{{2}}$
= 2.565, P > 0.05).
${{\chi}_{2}}^{{2}}$
= 2.565, P > 0.05).
Independent from our pheromone treatments, trap counts within the control plots fluctuated in all site years, ranging from zero to hundreds of males per week. These fluctuations, which we mainly observed at three-week intervals, were presumably due to multiple generations of midges at our experimental sites, which is typical in Ontario (Hallett et al. Reference Hallett, Goodfellow and Heal2007, Reference Hallett, Goodfellow, Weiss and Olfert2009b). Midge numbers also varied by week within the pheromone-treated plots but were dampened by the pheromone treatments. In 2016, local drought conditions strongly reduced midge emergence, and we observed only a few midges until the ninth week of the study; soil moisture is required for swede midge to complete pupation (Readshaw Reference Readshaw1966; Chen and Shelton Reference Chen and Shelton2007).
Plant damage and yield assessment
We found that the three-component treatments influenced the incidence of broccoli damage at six weeks and at the final damage assessment in 2017 (
![]() ${{\chi}_{2}}^{{2}}$
= 6.309, P = 0.043 and
${{\chi}_{2}}^{{2}}$
= 6.309, P = 0.043 and
![]() ${{\chi}_{2}}^{{2}}$
= 19.775, P < 0.001, respectively) but not in 2016 (Table 3). We did not observe significant differences in damage at three weeks (P > 0.05 for all models), likely due to lower midge populations at the beginning of the season and delayed onset of damage symptoms. In 2017, mean harvest damage ratings were more than three times higher in the control compared to in the stereospecific treatment plots (Table 3). Damage ratings were significantly lower in the stereospecific versus control plots, but damage did not significantly differ between either the control and racemic or the stereospecific and racemic plots.
${{\chi}_{2}}^{{2}}$
= 19.775, P < 0.001, respectively) but not in 2016 (Table 3). We did not observe significant differences in damage at three weeks (P > 0.05 for all models), likely due to lower midge populations at the beginning of the season and delayed onset of damage symptoms. In 2017, mean harvest damage ratings were more than three times higher in the control compared to in the stereospecific treatment plots (Table 3). Damage ratings were significantly lower in the stereospecific versus control plots, but damage did not significantly differ between either the control and racemic or the stereospecific and racemic plots.
Table 3. Mean swede midge damage ratings (± standard error of the mean) in broccoli at three and six weeks after transplanting and at harvest.
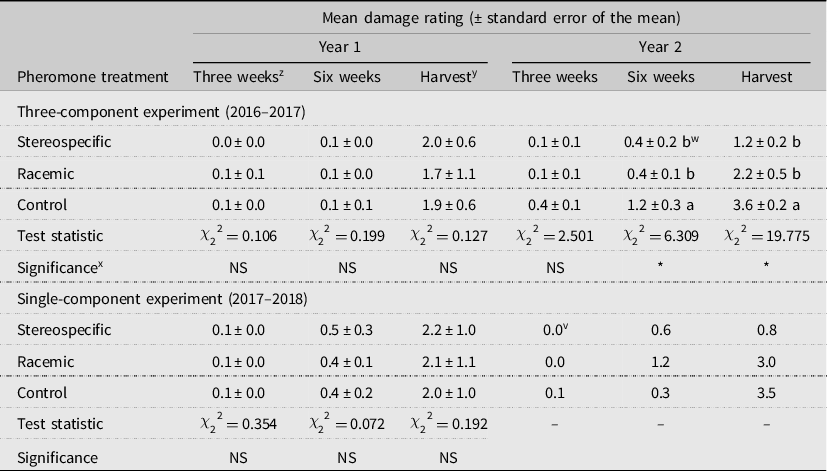
z Three- and six-week damage ratings were conducted using a four-point scale of vegetative damage, where “0” equals no swede midge damage, “1” equals mild twisting or scarring of petioles, leaves, and meristem swelling, “2” equals moderate or severe twisting or scarring of petioles, leaves, and meristem swelling, and “3” equals complete death of apical meristem.
y Harvest damage ratings were conducted using a six-point scale of increasing scarring of pedicels within the broccoli crown and accompanying deformity of head due to larval feeding, where “0” equals no damage, “1” equals mild scarring and deformity, “2” equals moderate scarring and deformity, “3” equals moderate or severe scarring and deformity, “4” equals severe scarring and deformity, and “5” equals complete death of apical meristem (no main broccoli crown).
x NS and * refer to nonsignificance (P > 0.05) and statistical significance at P < 0.05, respectively, of overall Kruskal–Wallis tests.
w Means indicated by different letters are statistically different (P < 0.05) according to Mann–Whitney U post hoc pairwise comparisons.
v Single-component damage rating means not followed by standard error of the mean due to one replicate in year 2 (2018).
Because damage ratings were lower in the three-component stereospecific plots, we observed a corresponding significant increase in marketable yields in 2017 (
![]() ${{{H}}_{2}}^{{2}}$
= 7.200, P = 0.027). Stereospecific treatments increased marketable heads ninefold from the nontreated control, from 6.7 ± 1.22% to 55.6 ± 4.59% marketable heads (Fig. 3). The percentage of marketable heads was numerically higher in the three-component racemic plots (25.0 ± 5.0%) than in the control plots. No significant differences were found among treatments in the percentage of marketable broccoli heads in our single-component experiment (pooled model: H
2 = 0.787, P > 0.05; Fig. 4).
${{{H}}_{2}}^{{2}}$
= 7.200, P = 0.027). Stereospecific treatments increased marketable heads ninefold from the nontreated control, from 6.7 ± 1.22% to 55.6 ± 4.59% marketable heads (Fig. 3). The percentage of marketable heads was numerically higher in the three-component racemic plots (25.0 ± 5.0%) than in the control plots. No significant differences were found among treatments in the percentage of marketable broccoli heads in our single-component experiment (pooled model: H
2 = 0.787, P > 0.05; Fig. 4).
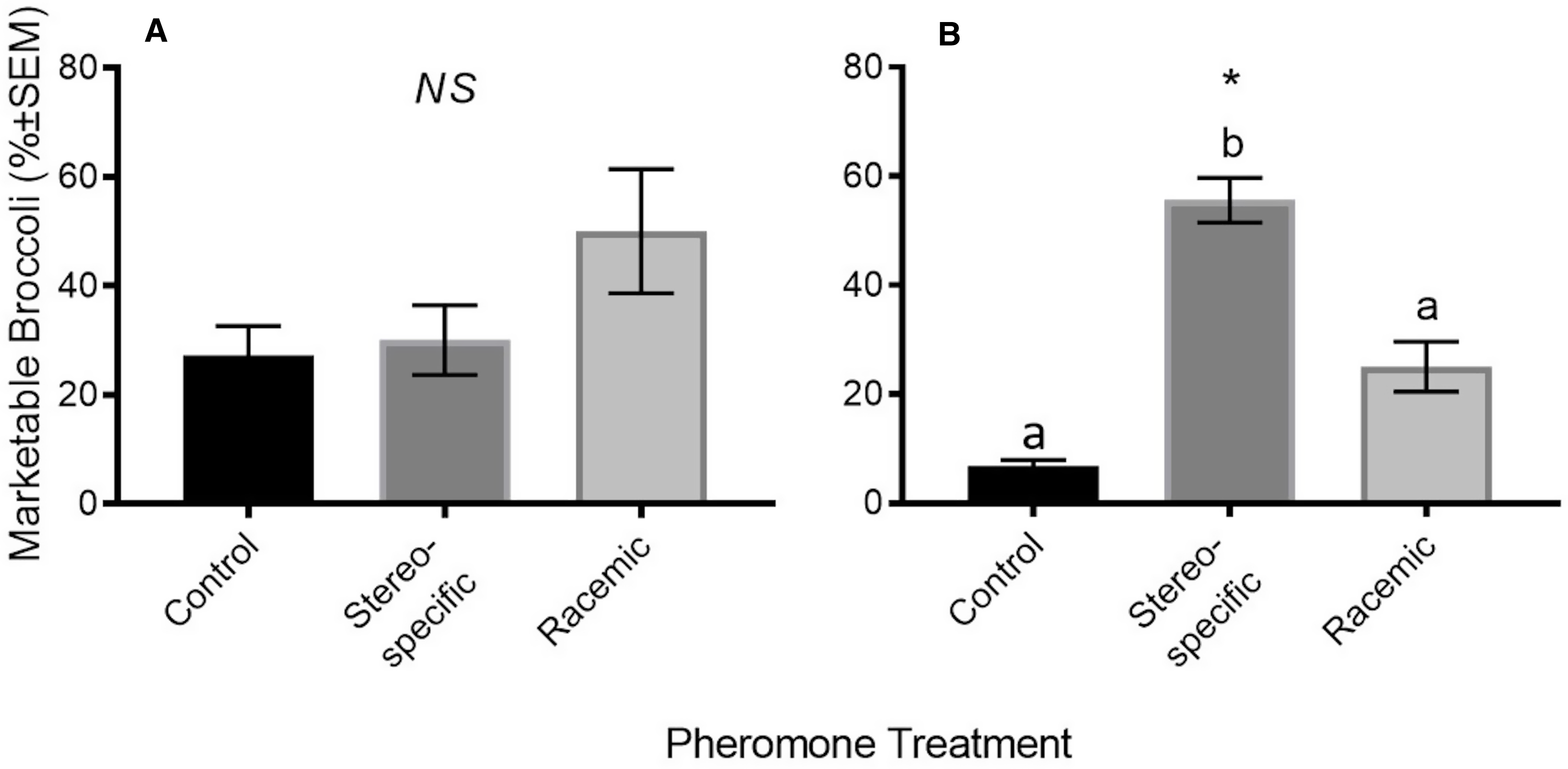
Fig. 3. Broccoli marketable yield in three-component experiment mating disruption plots in A, 2016 and B, 2017. NS indicates nonsignificant yield differences in 2016 (H 2 = 4.908, P > 0.05), and * indicates statistically significant yield differences in 2017 (H 2 = 7.200, P < 0.05). Treatments indicated by different letters are statistically different (P < 0.05).

Fig. 4. Broccoli marketable yield in single-component experiment mating disruption plots in A, 2017 and B, 2018. NS indicates nonsignificance (H 2 = 0.787, P > 0.05). The lack of error bars for 2018 is due to the presence of only one replicate in this year.
Discussion
Developing effective commercial pheromone-mediated mating disruption systems involves many years of experimentation to optimise pheromone blends and dispenser set-ups. Our research represents the first swede midge mating disruption study in North America. In the present study, we aimed to identify the most effective pheromone blend for mating disruption – a critical first step in mating disruption research and development. Despite the potential for cost savings by racemic and single-component pheromones, the three-component stereospecific blend is the most promising for swede midge mating disruption because this was the only treatment that resulted in significant trap shutdown and decreased swede midge damage in broccoli at harvest. Our results support the long-held hypothesis that the most attractive pheromone blend is the most effective for mating disruption (Minks and Cardé Reference Minks and Cardé1988; Cardé and Minks Reference Cardé and Minks1995).
Despite being the most promising treatment in our study, in 2017, the three-component stereospecific blend resulted in 44% unmarketable broccoli heads, which is unacceptably high for commercial farms. Mated females likely migrated into our plots, laying eggs in our treated broccoli. Migration of mated females is a common challenge for pheromone-mediated mating disruption in annual cropping systems, and it results in high levels of crop damage despite trap count reductions (Jiménez et al. Reference Jiménez, Toscano, Flaherty, Ilic, Zalom and Kido1988; Fadamiro et al. Reference Fadamiro, Cossé and Baker1999; Mori and Evenden Reference Mori and Evenden2015). Within annual crops, some of the most effective mating disruption systems have been used for tomato pests, particularly those within contained greenhouses (Vacas et al. Reference Vacas, Alfaro, Primo and Navarro-Llopis2011), where gravid females cannot enter the treated area. Otherwise, widespread commercial use of mating disruption for annual crops is limited due to high cost, female migration, overall inefficacy, and more suitable control alternatives (Welter et al. Reference Welter, Pickel, Millar, Cave, Van Steenwyk and Dunley2008; Miller and Gut Reference Miller and Gut2015).
The location of swede midge mating results in an increased likelihood of failed mating disruption when dispensers are set up outside of emergence sites. We have increasing evidence that swede midges mate immediately upon emergence (Hodgdon et al. Reference Hodgdon, Hallett, Stratton and Chen2019b), regardless of whether host plants are present. Mating at emergence sites and postmating migration also occur in other cecidomyiid pests in annual cropping systems, such as the brassica pod midge (Dasineura brassicae Winnertz) and orange wheat blossom midge (Sitodiplosis mossellana Géhin) (both Diptera: Cecidomyiidae) (Sylven Reference Sylven1970; Williams et al. Reference Williams, Martin and Kelm1987; Smith et al. Reference Smith, Wise and Lamb2007). If swede midges mate at their emergence sites and then migrate to host plants to oviposit, mating disruption treatments in the current year’s Brassica crops will be ineffective at providing crop protection, regardless of male disorientation and trap shutdown. Because midges overwinter in the soil and exhibit multiple emergence phenotypes, they often emerge over a prolonged time period from multiple previously cropped fields (Hallett et al. Reference Hallett, Goodfellow and Heal2007, Reference Hallett, Goodfellow, Weiss and Olfert2009b). Pheromone dispensers would be more useful if they were deployed at emergence sites. Further confirmation of swede midge mating location and migration patterns will be necessary to inform the installation of dispensers within a farm landscape.
We may have also experienced high levels of crop damage because of our small plot size. Milli et al. (Reference Milli, Koch and De Kramer1997) found significant variability in ambient pheromone levels within 10 m of treated field borders. Thus, pheromone-mediated mating disruption is more effective when larger areas are treated. Effective mating disruption systems in annual crops such as cotton were effective in preventing crop damage when large-scale applications (> 28 ha) limited “edge effects” (Staten et al. Reference Staten, Flint, Weddle, Quintero, Zarate and Finnell1987). We likely experienced significant edge effects due to our small plot size, with inadequate coverage of pheromone to suppress mating outside of the treated plots. Due to the resource-intensive nature of mating disruption experiments, achieving adequate replication using large-scale plots is challenging and often cost and space prohibitive. However, for swede midge, vegetable growers may need to treat larger tracts of land based on crop rotation history, which varies by farm. Larger, whole-farm proof-of-concept mating disruption experimental designs will be necessary to determine whether this tactic can be successful for swede midge management. Small-scale organic vegetable farms would be ideal candidates for demonstration.
Despite using a similar experimental design, Samietz et al. (Reference Samietz, Baur and Hillbur2012) did not observe high rates of crop damage in their swede midge mating disruption study in Europe. Swede midge damage in their treated Brussels sprouts (Brassica oleracea) test crops did not exceed 2%. However, swede midge populations were significantly lower at their test sites, possibly allowing for a greater reduction in crop damage. Their weekly mean trap counts never exceeded 50 males, and in control plots, damage never exceeded 20%. In the present study, we commonly observed more than 10 times the number of males per trap during weekly checks at peak emergence periods in July and August (Figs. 1, 2). We observed almost complete yield loss in several of our nontreated plots. For areas with higher swede midge pressure, a combination of management tactics, such as insecticides and mating disruption, may be necessary to sufficiently reduce damage. If mating disruption dispensers are needed for extended periods of time, in addition to other management tactics, in multiple fields, further research would be needed to reduce the cost of swede midge mating disruption for the system to be commercially feasible.
Although the cheaper single-component and racemic blends were unsuccessful, other methods can be used to decrease the cost of pheromone-mediated mating disruption, such as reducing dispenser densities and strategically turning off dispensers at certain times of the day. For example, deploying fewer yet more efficient dispensers could allow for labour savings associated with installation. Our dispenser density (80 dispensers per 16 × 16-m plot) was quite high and likely unrealistic for vegetable growers to implement. Aerosol “mega-dispensers” requiring fewer devices per unit area are economical and effective for some lepidopteran pests; aerosol dispensers can be programmed to release pheromone only during the times of day when insects are active (Rama et al. Reference Rama, Reggiori and Pratizzoli2002; Stelinski et al. Reference Stelinski, Gut, Haas, McGhee and Epstein2007; Higbee and Burks Reference Higbee and Burks2008; Casado et al. Reference Casado, Cave and Welter2014; Mori and Evenden Reference Mori and Evenden2015). Such devices are turned off when insects are naturally inactive, thus saving pheromone inputs. Swede midge exhibits diel periodicity of mating (Hodgdon et al. Reference Hodgdon, Hallett, Stratton and Chen2019b), which introduces the potential to turn off programmable aerosol dispensers during the afternoon and night when the insects are inactive.
Our field trials demonstrate the potential for pheromone-mediated mating disruption to contribute to an integrated pest management programme for swede midge. Future research and development efforts, specifically related to midge mating and migration patterns to inform optimal dispenser locations, will be necessary as next steps towards commercial adoption. Without knowledge of where midges mate, pheromone-mediated mating disruption for swede midge may not become commercially viable. Because crop rotation is not feasible for growers with small land bases and organic insecticides fail when midge populations are large (Seaman et al. Reference Seaman, Lange and Shelton2013; Evans and Hallett Reference Evans and Hallett2016), small-scale organic growers will benefit the most from a new ecologically based swede midge management strategy. The next logical steps include testing mating disruption for swede midge by installing dispensers at emergence sites based on cropping histories, paired with dispensers placed within Brassica crops.
As swede midge populations continue to build in North America, with the potential to spread to important vegetable-producing regions (Mika et al. Reference Mika, Weiss, Olfert, Hallett and Newman2008), effective management strategies are desperately needed to prevent economic losses on farms. Both our research results and Samietz et al.’s (Reference Samietz, Baur and Hillbur2012) study in Europe indicate the potential for effective swede midge pheromone-mediated mating disruption. Mating disruption may be most effective for swede midge in conjunction with other management strategies, such as insecticides, crop rotation, or netting, that reduce the population. Although mating disruption for lepidopteran pests, such as codling moth, has benefited from decades of research and development (Welter et al. Reference Welter, Pickel, Millar, Cave, Van Steenwyk and Dunley2008; Witzgall et al. Reference Witzgall, Kirsch and Cork2010), work on swede midge mating disruption is still in its early stages. With additional research, pheromone-mediated mating disruption could offer another tool for swede midge management.
Acknowledgements
Funding for this research was provided by the following sources: United States Department of Agriculture NIFA Crop Protection and Pest Management grant (2014-70006-22525) to Y.H.C., Northeast SARE Graduate Student grant (GNE16-121) to E.A.H., and the Ontario Ministry of Agriculture, Food and Rural Affairs University of Guelph Partnership to R.H.H. The authors are grateful for assistance from Kylie Barwise, Jamie Bauman, Jarrett Blair, Josée Boisclair, Thierry Boislard, Andrea Campbell, Charles-Étienne Ferland, Paolo Filho, Laurence Jochems-Tanguay, David Kells, David Laurie, Kate Lindsay, Charlotte MacKay, Ted Marois, Matthew Muzzatti, Cameron Ogilvie, Elizabeth Porter, Jorge Ruiz-Arrocho, Linley Sherin, Leah Slater, Emily Sousa, and Jonathan Williams from the Institut de recherche et développement en agroenvironnement, University of Guelph, and University of Vermont for the field trials. They also thank Pfenning’s Organic for providing land, technical assistance, and broccoli seedlings for the field trials at their farm. Lastly, the authors acknowledge assistance from Vincent Martineau in reviewing our French translation of the abstract.
Competing interests
The authors declare no competing interests.













