Introduction
Invasive alien species are organisms that are introduced accidentally or intentionally in areas outside their native range, and can spread at considerable rates, producing offspring (Traveset and Richardson, Reference Traveset and Richardson2006; Stout and Morales, Reference Stout and Morales2009) and causing harm to the environment, the economy (Diagne et al., Reference Diagne, Leroy, Vaissière, Gozlan, Roiz, Jarić, Salles, Bradshaw and Courchamp2021) or even human health (Bonanno, Reference Bonanno2016). Invasive species are recognised as one of the main threats to biodiversity (Kenis et al., Reference Kenis, Rabitsch, Auger-Rozenberg and Roques2007), and as important drivers of global environmental change (Sala et al., Reference Sala, Chapin, Armesto, Berlow, Bloomfield, Dirzo, Huber-Sanwald, Huenneke, Jackson, Kinzig, Leemans, Lodge, Mooney, Oesterheld, Poff, Sykes, Walker, Walker and Wall2000; Levine and D'Antonio, Reference Levine and D'Antonio2003). Although usually only a small proportion of alien species become invasive (Aizen et al., Reference Aizen, Arbetman, Chacoff, Chalcoff, Feinsinger, Garibaldi, Harder, Morales, Sáez, Vanbergen, Bohan and Vanbergen2020), it is often complicated to determine their ecological impact, because of a broad theoretical framework and the lack of an unambiguous definition that describes and quantifies such impacts (Ricciardi et al., Reference Ricciardi, Hoopes, Marchetti and Lockwood2013; Kumschick et al., Reference Kumschick, Gaertner, Vilà, Essl, Jeschke, Pyšek and Winter2015). This knowledge gap is thought to be particularly important for exotic pollinators, mainly because of difficulties in assessing the extent of niche overlap and competition with native species (Goulson, Reference Goulson2003).
Research on competition between native and exotic bees has mainly focused on managed species, and especially on the social bees Apis mellifera L., 1758 and Bombus terrestris (L., 1758) (Russo, Reference Russo2016). Both honeybees and bumblebees can prevent native bee species from foraging on the most abundant floral resources, overlapping their foraging niche and causing exploitative competition in areas where they have been introduced (Mallinger et al., Reference Mallinger, Gaines-Day and Gratton2017; Taggar et al., Reference Taggar, McGrath and Despland2021; Iwasaki and Hogendoorn, Reference Iwasaki and Hogendoorn2022; Paige and Williams, Reference Paige and Williams2023). In addition, these species can spread a large number of pathogens through flower visits (Graystock et al., Reference Graystock, Ng, Parks, Tripodi, Muñiz, Fersch, Myers, McFrederick and McArt2020; Cilia et al., Reference Cilia, Flaminio, Ranalli, Zavatta, Nanetti, Bortolotti and Bogo2023), representing another major threat to native bees (Morales et al., Reference Morales, Arbetman, Cameron and Aizen2013; Nanetti et al., Reference Nanetti, Bortolotti and Cilia2021). Although the majority of exotic bees are unmanaged solitary species, to date only a few studies have considered them, leaving a significant gap in knowledge about their relationships with native species and impact on ecosystems (Russo, Reference Russo2016; Graham et al., Reference Graham, Eaton, Obrien and Starks2019).
Among exotic bees, the family Megachilidae and the genus Megachile Latreille, 1802 in particular, are the most represented in terms of number of species (Russo, Reference Russo2016). Here we focused on the giant resin bee Megachile (Callomegachile) sculpturalis (Smith, 1958) (Hymenoptera: Megachilidae). Megachile sculpturalis is native to East Asia and occurs in countries of the South-East Asian coast, such as China, Korea, Taiwan and Japan (Iwata, Reference Iwata1933; Wu, Reference Wu2006). The common name ‘giant resin bee’ suggests two of the main characteristics of the species: its large body size and the use of plant resin as the main material for nest building and sealing (Batra, Reference Batra1998; Le Féon et al., Reference Le Féon, Aubert, Genoud, Andrieu-Ponel, Westrich and Geslin2018; Geslin et al., Reference Geslin, Gachet, Deschamps-Cottin, Flacher, Ignace, Knoploch, Meineri, Robles, Ropars, Schurr and Le Féon2020). Megachile sculpturalis is a univoltine cavity-nesting solitary bee that builds its nests in pre-existing cavities in wooden rods, dead wood or reed stems, as well as in nesting sites abandoned by a variety of other species and in artificial holes, including the so-called bee hotels (Iwata, Reference Iwata1933; Tsuneki, Reference Tsuneki1970; Guariento et al., Reference Guariento, Lanner, Staggi and Kranebitter2019). In non-native areas it can occasionally show aggressive behaviour towards the local bee fauna (Lanner et al., Reference Lanner, Huchler, Pachinger, Sedivy and Meimberg2020a), and its presence can be negatively correlated with the emergence of native bee species (Geslin et al., Reference Geslin, Gachet, Deschamps-Cottin, Flacher, Ignace, Knoploch, Meineri, Robles, Ropars, Schurr and Le Féon2020; Zakardjian et al., Reference Zakardjian, Jourdan, Le Féon, Geslin, Kevan and Willis Chan2022), suggesting potential competition for nesting sites. Brood cells are mainly composed by resin mixed with plant debris, while the closing plug is usually composed by a mixture of resin, mud and wood fibres (Batra, Reference Batra1998; Aguado et al., Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018). Due to its large dimensions, M. sculpturalis prefers cavities with entry diameters of at least 8 mm, although the size of the selected holes can vary according to their availability and to the size of females, which can display significant inter-individual variability (Geslin et al., Reference Geslin, Gachet, Deschamps-Cottin, Flacher, Ignace, Knoploch, Meineri, Robles, Ropars, Schurr and Le Féon2020). In Europe, the flight period of M. sculpturalis spans from mid-June to mid-September, with a peak in July (Geslin et al., Reference Geslin, Gachet, Deschamps-Cottin, Flacher, Ignace, Knoploch, Meineri, Robles, Ropars, Schurr and Le Féon2020).
The fact that M. sculpturalis nests in pre-existing cavities in wood, rods and reed stems, which are easily available and accessible in several natural and anthropic habitats, is supposed to facilitate its dispersal across countries and continents, likely favoured by the international trade in wood for commercial purposes (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014; Bortolotti et al., Reference Bortolotti, Luthi, Flaminio, Bogo and Sgolastra2018; Lanner et al., Reference Lanner, Huchler, Pachinger, Sedivy and Meimberg2020a, Reference Lanner, Dubos, Geslin, Leroy, Hernández-Castellano, Dubaić, Bortolotti, Calafat, Ćetković, Flaminio, Le Féon, Margalef-Marrase, Orr, Pachinger, Ruzzier, Smagghe, Tuerlings, Vereecken and Meimberg2022). The first record of M. sculpturalis outside its native range occurred in the 1990s in North Carolina, USA (Mangum and Brooks, Reference Mangum and Brooks1997), from where it likely expanded rapidly into the north-eastern states and Canada (Hinojosa-Díaz et al., Reference Hinojosa-Díaz, Yáñez-Ordóñez, Chen, Peterson and Engel2005; Hinojosa-Díaz, Reference Hinojosa-Díaz2008; O'Brien and Craves, Reference O'Brien and Craves2008; Parys et al., Reference Parys, Tripodi and Sampson2015). In Europe, M. sculpturalis was first observed in 2008 in France, near the port of Marseille (Vereecken and Barbier, Reference Vereecken and Barbier2009), from where it likely spread to Italy in 2009 (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014). It has subsequently been recorded in several European countries, likely as a result of both expansion and multiple independent introductions (Lanner et al., Reference Lanner, Gstöttenmayer, Curto, Geslin, Huchler, Orr, Pachinger, Sedivy and Meimberg2021), and potential further expansion has been predicted based on both climate and anthropogenic factors (e.g. Polidori and Sánchez-Fernández, Reference Polidori and Sánchez-Fernández2020; Lanner et al., Reference Lanner, Dubos, Geslin, Leroy, Hernández-Castellano, Dubaić, Bortolotti, Calafat, Ćetković, Flaminio, Le Féon, Margalef-Marrase, Orr, Pachinger, Ruzzier, Smagghe, Tuerlings, Vereecken and Meimberg2022).
In its native areas, M. sculpturalis is polylectic and forages on plants such as Pueraria lobata (Willd.) Ohwi in Japan and Lagerstroemia indica L. in China (Batra, Reference Batra1998; Mangum and Sumner, Reference Mangum and Sumner2003). Analyses on pollen provisions in non-native areas suggest that pollen collection occurs mainly on Styphnolobium japonicum (L.) Schott (Westrich et al., Reference Westrich, Knapp and Berney2015; Aguado et al., Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018; Andrieu-Ponel et al., Reference Andrieu-Ponel, Ponel, Le Féon, Geslin and Duvallet2018), which in many cases accounts for 100% of the provision, and to a lesser extent on Ligustrum sp. (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014; Andrieu-Ponel et al., Reference Andrieu-Ponel, Ponel, Le Féon, Geslin and Duvallet2018). These plants are both native to Asia and are often used as ornamental plants in Europe. On the contrary, nectar gathering is achieved on several native and exotic plant species (e.g. Guariento et al., Reference Guariento, Lanner, Staggi and Kranebitter2019; Ruzzier et al., Reference Ruzzier, Menchetti, Bortolotti, Selis, Monterastelli and Forbicioni2020).
Although some aspects of the nesting biology of M. sculpturalis in invaded areas have been investigated (e.g. progeny weight, sex ratio; Straffon-Díaz et al., Reference Straffon-Díaz, Carisio, Manino, Biella and Porporato2021), little is still known on nesting behaviour, nest structure and pollen preference of M. sculpturalis in non-native areas. To address such knowledge gaps, we have followed bees visiting artificial nests in a bee hotel in northern Italy for three consecutive years. We recorded the flight period of both males and females, and followed single individuals throughout their activity period to investigate nesting behaviour. We then inspected the nests to provide detailed information on the size and architecture of the various components. In addition, we analysed pollen provisions collected by M. sculpturalis and co-occurring native species in the same nesting structure, with the aim of evaluating their pollen preference and assessing possible niche overlap and potential competition for foraging and nesting resources.
Materials and methods
Study site
This study was conducted during three consecutive years, from 2016 to 2018, on a bee hotel (40 × 70 × 150 cm) located in the garden of the Research Centre for Agriculture and Environment (CREA-AA) in Bologna, Italy (44°31′26.9″N, 11°21′05.3″E) (Supplementary fig. 1). The bee hotel was installed in 2014 and was first visited by M. sculpturalis in 2016. It contained several different kinds of nests: solid wood cubes presenting cavities of 10 cm in length and diameters from 0.6 to 1.4 cm; hardboard cubes containing 28 holes each of 15 cm in length and seven different diameters, ranging from 0.2 to 1.4 cm; trunk segments from local tree species (walnut, elm, oak and cypress) presenting cavities of 11–12 cm in length and diameters ranging from 0.4 to 1.0 cm. In 2017, after all males and females of M. sculpturalis emerged, access to the trunk segments and cubes was excluded by fine-mesh nets to prevent further nesting, and only cut reeds (Arundo donax Forssk.) were provided as nesting sites. Finally, in 2018 all previously described nest types were left available for nesting by all bee species. Beside M. sculpturalis, several hymenopteran species nested in the bee hotel, although they only occupied less than 5% of the available nests: in spring, mason bees (Osmia cornuta and O. bicornis), and potter wasps (probably genus Ancistrocerus); in summer, Ancistrocerus spp. wasps, the native bees Heriades truncorum and Anthidium sp., and the exotic bee M. disjunctiformis.
During the flight period of M. sculpturalis, there were several flowering plant species available in the garden as potential food resources: one individual of Passiflora caerulea L., one Catalpa bignonioides Walter tree, three Vitex agnus-castus L. shrubs and a few shrubs of Linnaea chinensis A. Braun and Vatke. In addition, within a radius of 500 m around the garden, there were two allotment gardens of 0.9 and 0.4 ha, respectively, and one public park of approximately 7 ha, hosting ten S. japonicum trees and a small population of Cymbalaria muralis G. Gaertn, B. Mey and Schreb (Supplementary fig. 1). Private gardens around the area hosted few individuals of L. indica, V. agnus-castus, Ligustrum spp., Punica granatum L. and Diospyros kaki. More flowering plant species, including ornamentals, were likely available in private gardens.
Flight period and nesting behaviour of M. sculpturalis
Each year of the study, we recorded the first and last day on which males and females of M. sculpturalis were observed. In addition, in 2017 and 2018, we counted and marked (with a water-based colour to avoid double-counting) every day all new females that visited or nested in the bee hotel. Individuals for which the markings were fading were marked again to avoid confusion with new individuals. To relate climate parameters to the flight period of adults, daily mean temperatures and precipitations in Bologna from June to September in the 3 years of study were obtained by the ARPAE (Regional Environmental Protection Agency) database (https://dati.arpae.it/dataset/dati-meteoclimatici-comunali).
At the end of the 2017 and 2018 bee activity seasons, we counted and marked (with different colours each year) all the occupied (i.e. closed) cavities (hereafter ‘nests’, regardless of whether they occurred in reeds, wood blocks or trunk segments). In both 2017 and 2018, we estimated the mean number of nests completed per female by dividing the total number of nests in the bee hotel by the total number of females observed nesting. In addition, in 2018, a total of 41 females of M. sculpturalis were individually marked (with water-based colours) and observed daily for at least three consecutive hours, throughout their life, to determine the number of nests and the time taken to complete them. These females were marked on the thorax with a combination of two colours, and all the nests were marked with the same colours and counted at the end of their flight period. We recorded the time elapsed from the first entry into a new cavity until its closure. We also recorded the number of nest usurpations (i.e. a nest started by one female but then occupied and finished by another female of M. sculpturalis) in which each of these females were involved, by checking daily the correspondence between female and nest colours.
Nest structure
At the end of the nesting period in 2017, we collected and inspected 100 nests randomly chosen among the reeds of the bee hotel to investigate nest structure. In each nest, we recorded the cavity diameter, number and length of chambers, presence of brood, any anomalies and collected pollen samples where available (fig. 1). We considered as first chamber the one closest to the entrance (i.e. the last chamber of the nest built by females). In addition, each year at the end of the nesting season, we counted all closed cavities in the bee hotel and measured their diameter.
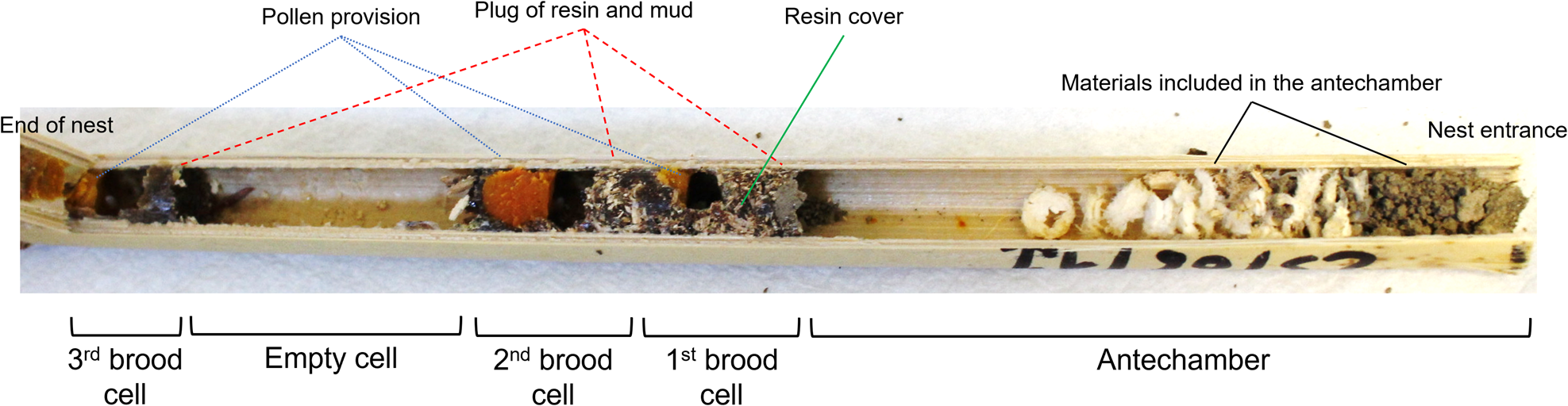
Figure 1. Structure of a typical Megachile sculpturalis nest, with details on the number and type of chambers and their contents. Antechamber is a chamber without brood sited before the first brood cell.
Palynological analyses
In the 3 years of study, we collected 198 pollen samples from brood cells in 139 nests completed by M. sculpturalis. In 2016 and 2017, we also collected 14 pollen samples belonging to six nests of the native bee Anthidium sp. (Hymenoptera, Megachilidae). All pollen samples (n = 212) were virtually intact, belonging to provisions with no or negligible larval feeding activity, and were analysed to determine their botanical origin (qualitative analysis). Each sample was diluted in 10 ml of distilled water, then the solution was stirred until the pollen mass was completely dissolved. An aliquot of the obtained solution was placed on a microscope slide and observed at 400 × magnification under an optical microscope (Zeiss Axiolab re). From each sample, 500 randomly chosen pollen grains were observed and identified at the lowest possible taxonomic level.
To estimate the total number of pollen grains forming pollen loads (quantitative analysis), we collected a second aliquot of 10 μl only from samples showing fully intact pollen loads (n = 39), due to missing, dead or unhatched eggs. The procedure was the same as for the qualitative analysis, except here we counted all the pollen grains present on the slides, and the values obtained were multiplied by 103 to estimate the total amount in the original sample.
We calculated the mean volume of pollen grains by measuring the equatorial and polar radii of 60 randomly chosen pollen grains of S. japonicum found in pollen loads, using an ocular micrometre. Subsequently, we calculated their mean volume treating them as spheroids with volume = 4/3(π)(a 2)(b), where a = equatorial radius and b = polar radius (O'Neill and O'Neill, Reference O'Neill and O'Neill2011). Then, we calculated the mean volume of pollen loads by multiplying the mean volume of pollen grains by the mean number of grains present in the pollen loads analysed.
We collected 35 flowers of S. japonicum in different anthesis stages from five plants in the vicinity of CREA-AA, to estimate the number of pollen grains present in single flowers (Müller et al., Reference Müller, Diener, Schnyder, Stutz, Sedivy and Dorn2006). We analysed five groups of seven flowers separately. All flowers from each group were immersed in a tube filled with ether for 5 min, then stirred for 2 min and sonicated for 1 min at 40 kHz. Subsequently, ether was evaporated completely by nitrogen flow, 10 ml of distilled water and a drop of 0.1% ethanol solution of basic fuchsine were added, and the newly obtained solution was stirred for 1 min. The solution was then filtered by vacuum filtration apparatus using a membrane filter of mixed cellulose esters with pore size of 1.2 μm and filtration surface diameter of 34 mm. Filters were then dried on a plate heated at 40°C for 5–10 min until completely dry and mounted on a microscope slide with cedar oil for microscopy. Finally, we counted the absolute number of pollen grains following the method described by Von Der Ohe et al. (Reference Von Der Ohe, Persano Oddo, Piana, Morlot and Martin2004).
Data analysis
The mean number of nests completed per female in 2017 and 2018 were compared by means of a Pearson's χ2 test. To evaluate variation in antechamber (i.e. chamber without brood sited before the first brood cell) length, we fitted a linear model using antechamber length as response variable, and total nest length and nest diameter as predictors. Because larvae, and consequently adults, of M. sculpturalis have highly variable body size (Stock et al., Reference Stock, Piot, Vanbesien, Meys, Smagghe and De Baets2021), we assessed variation in brood cell length by fitting a linear model using log-transformed brood cell length as response variable, and total nest length, nest diameter, antechamber length and number of brood cells as predictors. To evaluate the dependence of the number of brood cells produced by females on multiple nest variables, we fitted a generalised linear model (R package ‘glmmTMB’; Brooks et al., Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Maechler and Bolker2017) with Conway–Maxwell Poisson error distribution (Huang, Reference Huang2017) and log-link function, using brood cell number as response variable, and total nest length, nest diameter and antechamber length as predictors. Multicollinearity between predictors in each model was estimated through variance inflation factors (VIFs) using the R package ‘performance’ (Lüdecke et al., Reference Lüdecke, Ben-Shachar, Patil, Waggoner and Makowski2021): all VIFs were <1.5, indicating absence of collinearity. Distribution of model residuals was checked using the R package ‘DHARMa’ (Hartig, Reference Hartig2022). All analyses were performed in R v4.1.1 (R Core Team, Reference R Core Team2021). Means are reported ± standard error (SE).
Results
Flight period and nesting behaviour
The first individual of M. sculpturalis to ever visit the bee hotel was a female observed on 5 July 2016. Over the 3 years of study, the date of first visit by females varied little, occurring along a two-and-a-half week span, while the last visit date varied widely across years, spanning almost 9 weeks (fig. 2a). In 2016, we recorded the longest visitation period (77 days), in 2017 the shortest (31 days), and in 2018 an intermediate period (59 days, fig. 2a). Males of M. sculpturalis started visiting bee hotels on 12 June both in 2017 and 2018, and their visitation period lasted 15 days in 2017 and 18 days in 2018 (fig. 2a).
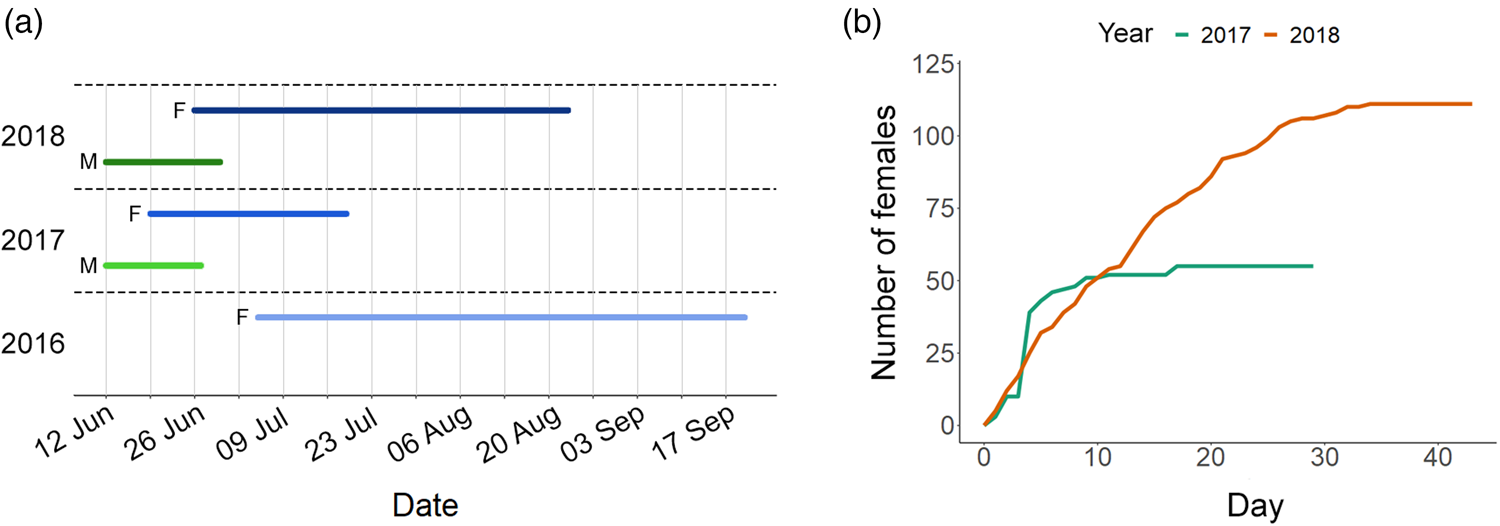
Figure 2. (a) Activity period of M. sculpturalis females (F) and males (M) in the bee hotel in the 3 years of study, and (b) cumulative number of new female individuals of M. sculpturalis that visited the bee hotel in 2017 and 2018, starting from the first day (day 0) in which females were observed. In 2016, we did not observe any males, because only mated females arrived to nest in the bee hotel.
The number of females that visited and nested in the bee hotel increased from 55 in 2017 to 111 in 2018. In 2017, more than 50% of females that visited the bee hotel arrived on the same day (26 June), while more than 70% arrived in the first week (fig. 2b). In 2018, the arrival of new females was more spread-out throughout the activity period (fig. 2b).
When considering climatic parameters, mean monthly temperatures were higher and precipitation was lower in 2017 than in 2016 and 2018 (Supplementary table 1). In addition, in September 2017, there was a sudden drop in temperatures associated with a peak in precipitation.
In 2018, we were able to record the number of nests built by 26 individual females, and the time required for nest completion for 23 of them. On average, females took 2.52 ± 0.17 days to complete a nest (range: 1–5 days, n = 50 nests). The mean number of nests completed per female was 4.27 ± 0.54 (n = 111 nests). The mean number of nests completed by all females that visited the bee hotel was significantly higher in 2017 (mean = 4.51, 248 nests/55 females) than in 2018 (mean = 2.24, 249 nests/111 females; χ2 = 14.097, P < 0.001). In addition, we recorded a total of 17 nest usurpations performed by 11 females of M. sculpturalis, and we frequently observed other antagonistic behaviours among nesting females, such as fights and robbing of nesting materials (Supplementary fig. 2). We also recorded aggressive interactions with another exotic Megachile species, M. disjuntiformis Cockerell 1911 (Supplementary fig. 3), that in 2018 built about five nests in the bee hotel, but not with other hymenopteran species (Ancistrocerus spp., H. truncorum and Anthidium sp.) that nested in the same bee hotel in the same months in 2017 and 2018.
Nest structure
We measured cavity diameter in 55 nests in 2016, and in all completed nests in 2017 and 2018 (n = 248 and n = 249, respectively), for a total of 552 nests. The mean nest diameter was 0.85 ± 0.01 cm, with almost 50% of the diameters between 0.8 and 1.00 cm and more than 95% between 0.6 and 1.2 cm (fig. 3).

Figure 3. Number of completed nests per diameter class recorded throughout the study period (n = 552).
Of the 100 reeds inspected, five were closed by a plug of resin and mud but were completely empty inside and were therefore excluded from the analysis. In addition, 15 nests presented the plug followed by a single brood cell as long as the entire cavity. The remaining 80 nests presented a first chamber (i.e. the antechamber, before the first brood cell), closed on the outside with a plug made of mud and resin, containing various materials such as lint, bits of wood, mud and remnants of soft reed septi (fig. 1). The antechamber did not contain brood or pollen and had an average length of 10.34 ± 0.44 cm (range 2.1–19.5 cm). Antechamber length significantly increased with increasing total nest length (fig. 4), while it did not depend on nest diameter (Supplementary table 2).

Figure 4. Positive relationship between antechamber length and nest length in the 80 nests inspected in 2017. The blue line represents the best linear regression fit, and shaded areas report 95% confidence intervals.
In the 80 nests presenting an antechamber, we counted a total of 148 brood cells. On average, brood cells were 2.85 ± 0.13 cm long (range 1.1–12 cm). Brood cell length significantly decreased with increasing number of brood cells (fig. 5) and close-to-significantly decreased with increasing antechamber length (P = 0.05), while it was not dependent on nest length or diameter (Supplementary table 3). Nests contained an average of 1.85 ± 0.11 brood cells (range 1–4). The number of brood cells per nest significantly increased with increasing nest length and diameter, and decreased with increasing antechamber length (fig. 6, Supplementary table 4).

Figure 5. Negative relationship between length of brood cells (cm) and the number of brood cells produced by females in the 80 nests inspected in 2017. The blue line represents the best linear regression fit, and shaded areas represent 95% confidence intervals.
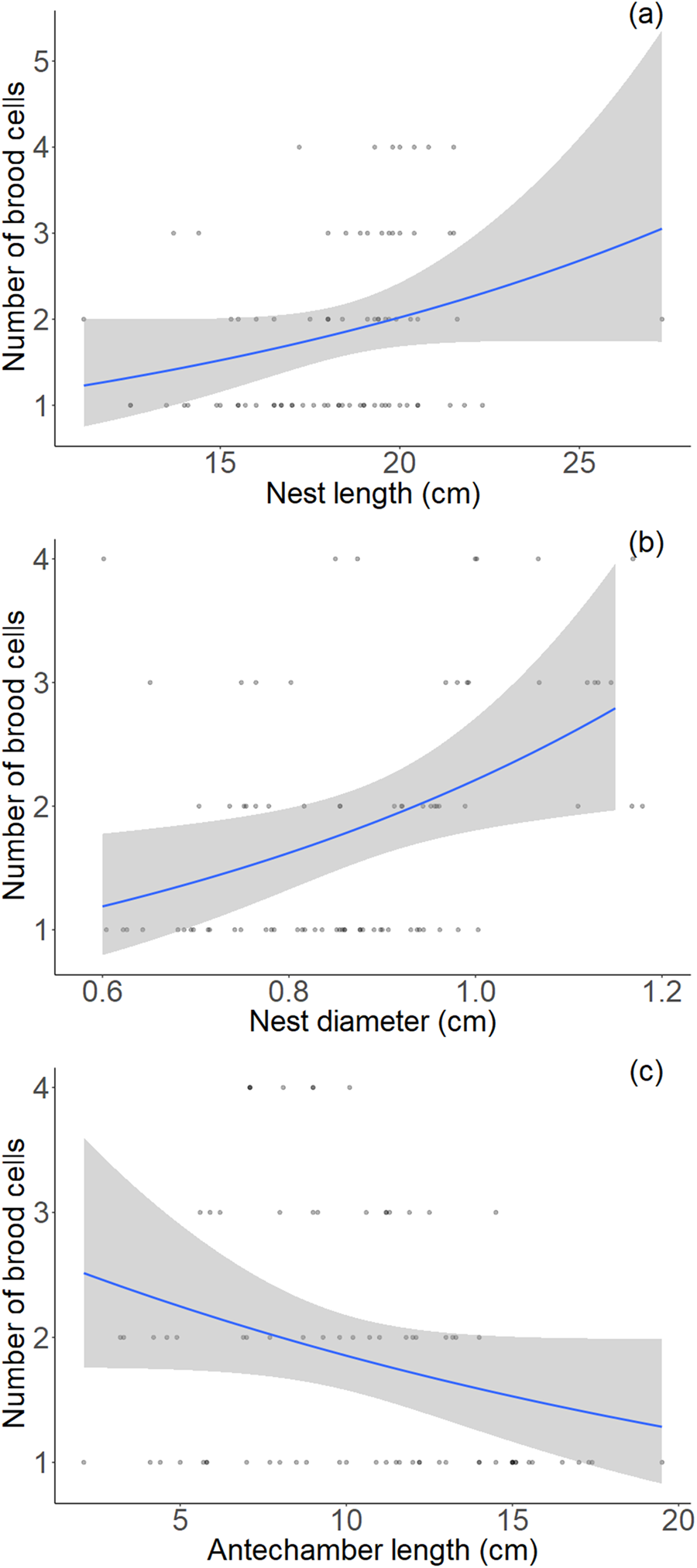
Figure 6. Relations between the number of brood cells completed by females in 2017 and (a) nest length, (b) nest diameter and (c) antechamber length (n = 80 nests). Blue lines represent the best linear regression fit, and shaded areas represent 95% confidence intervals. Raw data points were jittered to avoid overlap.
Seventeen of the 80 nests containing an antechamber had one or two empty chambers (19 empty chambers in total), located between the antechamber and the first brood cell or between two brood cells (fig. 1).
In two cells, we found the presence of the beetle Trogoderma glabrum (Herbst, 1783), a parasite commonly associated with nests of other Megachilidae (Zhantiev, Reference Zhantiev2009).
Palynological analyses
The qualitative palynological analysis performed on 212 pollen samples showed that almost all the pollen collected by M. sculpturalis (99.99 ± 0.01%) and Anthidium sp. (97.17 ± 1.46%) belonged to the plant S. japonicum (table 1). Pollen provisions of S. japonicum presented two different colourings, yellow and orange (Supplementary fig. 4), something that to our knowledge has never been reported before (Simonetti et al., Reference Simonetti, Frilli, Barbattini and Iob1989; Ricciardelli D'Albore, Reference Ricciardelli D'Albore1998). Only four samples collected from nests of Anthidium sp. in 2016 showed more than five pollen grains (>1%) belonging to other plant species (i.e. L. indica, Compositae sp., Medicago sp., Rubus fruticosus Marshall, V. agnus-castus, Trifolium pratense L., Brassicaceae sp.).
Table 1. Percentage of pollen grains of Styphnolobium japonicum found in pollen samples retrieved from nests of Megachile sculpturalis and Anthidium sp. over the 3 years of study

Nc, number of brood cells; Nn, number of nests.
The quantitative analysis performed on 39 samples collected from nests of M. sculpturalis showed that pollen loads were composed on average by 1.42 × 106 ± 0.15 × 106 pollen grains. The mean volume of a single pollen grain of S. japonicum was 1935.74 ± 51.33 μm3, and the mean total volume of pollen loads was 2.74 ± 0.30 mm3.
The mean number of pollen grains per S. japonicum flower was 8130.54 ± 759.73, indicating that females of M. sculpturalis should visit on average 179.1 ± 13.72 flowers to collect the pollen needed to complete a single brood cell.
Discussion
Flight period and nesting behaviour
Our results on flight period are consistent with data collected in all the European countries where M. sculpturalis is present, ranging from mid-June to mid-September with a peak in July (Straffon-Díaz et al., Reference Straffon-Díaz, Carisio, Manino, Biella and Porporato2021), with males emerging 1–2 weeks earlier than females (Kakutani et al., Reference Kakutani, Inoue, Kato and Ichihashi1990). However, unlike the start and end times of males, which were fairly stable among years, the start and end of female activity were highly variable. This inter-annual variability at the beginning of the flight period may be related to climatic parameters, such as temperature and precipitation during the flight period (Vergara et al., Reference Vergara, Fierro, Carvajal, Alaniz, Zorondo-Rodríguez, Cifuentes and Castro2023). Among the 3 years of study, June 2017 was the hottest and coincided with an earlier beginning of female activity compared to the other years. Temperatures in June 2018 were intermediate and those in 2016 were the lowest, and coincided with intermediate and later onset of female activity, respectively. The variability of the duration and end of flight periods among years could be explained by food availability in the vicinity of the nesting sites (Dubaić et al., Reference Dubaić, Plećaš, Raičević, Lanner and Ćetković2022), or by intrinsic characteristics of the invading population (Bortolotti et al., Reference Bortolotti, Luthi, Flaminio, Bogo and Sgolastra2018). Moreover, the higher temperatures recorded in the summer of 2017 might have affected the life cycle of females, restricting their emergence and activity to a limited period of time with suitable weather conditions, and have likely reduced the flowering period of plants on which M. sculpturalis forages, leaving resources available for shorter time.
We recorded a significant decrease in the number of nests completed by females from 2017 to 2018, although the number of females visiting nests was considerably higher in 2018. Such difference was likely related to higher intra-specific competition due to the higher density of females nesting in the bee hotel. The fact that there were twice as many females in 2018 than in 2017 while the number of nests remained stable suggests that part of the nesting females had left the bee hotel to seek other nesting sites. Interestingly, females individually observed in 2018 had comparable nesting rates of 2017 females, suggesting that individuals that successfully nest in the bee hotel have comparable nesting rates among years independently from the level of intra-specific competition. In addition, the constant nesting rate between years suggests a weak dependence of the single nesting female on climatic conditions in the study area once the nesting activity has started.
Nest structure
According to previous studies performed on a limited number of nests, M. sculpturalis requires cavities at least 8 mm in diameter, and nests include brood cells constructed separately for each individual offspring (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014; Straffon-Díaz et al., Reference Straffon-Díaz, Carisio, Manino, Biella and Porporato2021; Dubaic and Lanner, Reference Dubaić and Lanner2021). Our results on 552 nests were partially consistent with the literature, with a mean diameter of 0.85 cm. However, nests showed high variability with almost 50% of the diameters between 0.8 and 1.00 cm, and more than 95% between 0.6 and 1.2 cm. Interestingly, 25% of nests had small diameters of less than 0.8 cm, a nest size never observed before for M. sculpturalis. Most of the 100 nests inspected presented a first chamber (i.e. the antechamber) without brood. The antechamber was very variable in length, and often contained various materials such as lint and pieces of wood or mud. The antechamber, also called ‘vestibular cell’, was described as a very common feature of Aculeata nests by Krombein (Reference Krombein1967). It has been interpreted as a risk-spreading strategy, aimed at discouraging nest penetration by parasites or predators (Krombein, Reference Krombein1967; Michener and Brooks, Reference Michener, Brooks, Melo and Alves-dos-Santos2003; Morato and Martins, Reference Morato and Martins2006). In our study, antechambers were generally followed by an average of about two brood cells, with an average length of 2.5 cm each. These findings are consistent with the two nests described by Ivanov and Fateryga (Reference Ivanov and Fateryga2019), where brood cells occupied only a small portion of the cavity, although the authors did not mention the presence of an antechamber.
We found that females of M. sculpturalis tend to build more nests with fewer cells, rather than building more cells in fewer nests, compared to other species of the same family that show similar nesting behaviour and nest structure. For example, Osmia bicornis (Linnaeus, 1758) has been found to build about six brood cells per nest and less than two nests in total (Fliszkiewicz et al., Reference Fliszkiewicz, Kuśnierczak and Szymaś2012), while Megachile rotundata (Fabricius, 1787) had an average of 4–6 brood cells within a single built nest (Pitts-Singer and Bosch, Reference Pitts-Singer and Bosch2010). The behaviour observed in M. sculpturalis can be explained as a strategy to reduce offspring loss in case of nest usurpation or in case of attacks from parasites. In addition, when nests were longer females built longer antechambers but fewer brood cells. Such behaviour may also be related to a decreased risk of losing offspring in each nest, suggesting that the nesting strategy of M. sculpturalis favours safety over space exploitation. Because there were many more nests available in the bee hotel studied than were needed by females, as many nests were not used, it would be interesting to investigate whether this behaviour would remain the same if nests were limited.
We found a considerable number of nests (n = 37) with anomalies. Some nests presented an empty cavity closed with mud, some a single brood cell as long as the entire cavity, and some an empty chamber situated after the antechamber. The latter was named ‘intercalary cell’ by Krombein (Reference Krombein1967), and their presence was described as a behavioural response to external factors such as parasites, mould spores or other contaminants.
Palynological analyses
This is the first study to analyse a considerable number of pollen samples retrieved from nests of M. sculpturalis (n = 198). To our knowledge, only four other studies (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014; Westrich et al., Reference Westrich, Knapp and Berney2015; Andrieu-Ponel et al., Reference Andrieu-Ponel, Ponel, Le Féon, Geslin and Duvallet2018; Aguado et al., Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018) have performed palynological analyses on M. sculpturalis, on a total of three samples from brood cells and seven samples from female scopae. All other studies on food preference have investigated M. sculpturalis–plant interactions (e.g. Mangum and Sumner, Reference Mangum and Sumner2003; Ruzzier et al., Reference Ruzzier, Menchetti, Bortolotti, Selis, Monterastelli and Forbicioni2020; Ribas-Marquès and Díaz-Calafat, Reference Ribas-Marquès and Díaz-Calafat2021). We found that S. japonicum, a common ornamental tree native to the home range of M. sculpturalis, was the main and almost the only pollen source for larval provision. This result is in accordance with the previously cited studies (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014; Westrich et al., Reference Westrich, Knapp and Berney2015; Andrieu-Ponel et al., Reference Andrieu-Ponel, Ponel, Le Féon, Geslin and Duvallet2018; Aguado et al., Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018), except for one sample from a brood cell (Quaranta et al., Reference Quaranta, Sommaruga, Balzarini and Felicioli2014) and one from a female scopa (Aguado et al., Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018), that contained mostly pollen from Ligustrum sp. Our results support the relevance of S. japonicum as the most important food plant for M. sculpturalis outside its native range. Thus, S. japonicum could potentially be used for early-phase colonisation monitoring, as suggested by Dubaić et al. (Reference Dubaić, Plećaš, Raičević, Lanner and Ćetković2022), or even as a control method by reducing or ceasing its use as an ornamental plant. Based on our results on the number of cells and nests produced, and the number of flowers visited to complete a single brood cell, we estimated that each female M. sculpturalis can visit more than 1400 flowers throughout her adult life for pollen provisioning. Such foraging effort is likely to be beneficial, as it can be expected to be positively correlated with increased offspring production. However, long periods away from the nest may increase the likelihood of nest usurpation or offspring parasitisation (Goodell, Reference Goodell2003), potentially explaining the great importance given by this species to nest protection via the antechamber and the low nest:brood cells ratio.
The total volume of pollen grains found in the larval provisions was less than 3 mm3. The gap with the remaining volume is likely attributable to empty space among pollen grains, and mainly to other provision constituents, such as nectar. In fact, previous studies found that in other Megachilidae species nectar could represent two-thirds, or even more, of the pollen provision's mass (Strickler, Reference Strickler1979, Reference Strickler1982; Neff, Reference Neff2008; Cane et al., Reference Cane, Gardner and Harrison2011).
Niche overlap and potential inter- and intra-specific competition
The analysis of the 14 brood cells produced by Anthidium sp. showed an almost complete overlap with the pollen collected by M. sculpturalis, indicating that the two species may compete both for larval food resources and for nesting sites. Further competition for nesting sites and nesting material between M. sculpturalis and native bee species can be hypothesised based on the size of the nesting cavities occupied. Because females of M. sculpturalis did not present a strong selectivity in nest size, nesting in cavities between 0.6 and 1.2 cm in diameter, they overlap with many other species of cavity-nesting Apoidea (Batra, Reference Batra1998; Mangum and Brooks, Reference Mangum and Brooks1997; Zandigiacomo and Grion, Reference Zandigiacomo and Grion2017; Aguado et al., Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018). Some studies found evidence for nesting competition. For example, Geslin et al. (Reference Geslin, Gachet, Deschamps-Cottin, Flacher, Ignace, Knoploch, Meineri, Robles, Ropars, Schurr and Le Féon2020) showed that as soon as a single female of M. sculpturalis arrived at a nesting site, the rate of native bees dropped by 51%; Aguado et al. (Reference Aguado, Hernández-Castellano, Bassols, Miralles, Navarro, Stefanescu and Vicens2018) reported the use by M. sculpturalis of the same nesting resources as the megachilid Anthidium florentinum (Fabricius, 1775); and Le Feon and Geslin (Reference Le Féon and Geslin2018) observed females of M. sculpturalis emptying the content of nests of O. bicornis, O. cornuta (Latreille, 1805) and Isodontia mexicana (de Saussure, 1867) to build their own. We observed M. sculpturalis females robbing nest materials from occupied or previously used empty nests of other species (personal observation). We also recorded aggressive interactions with another exotic Megachile species, M. disjuntiformis, which occupied few nests in the same bee hotel (Bortolotti et al., Reference Bortolotti, Luthi, Flaminio, Bogo and Sgolastra2018). Other studies reported aggressive, even lethal behaviour by M. sculpturalis against native species, such as Xylocopa virginica (L., 1771) and H. truncorum (L., 1758) (Laport and Minckley, Reference Laport and Minckley2012; Roulston and Malfi, Reference Roulston and Malfi2012; Lanner et al., Reference Lanner, Meyer, Harmetzky, Meimberg and Pachinger2020b).
The antagonistic behaviours among nesting females we observed (such as fights, nest usurpations and robbing of nesting materials) are known from a wide variety of solitary aculeates (Field, Reference Field1992), including megachilid bees (Raw, Reference Raw1972; Eickwort, Reference Eickwort1975; Tepedino and Torchio, Reference Tepedino and Torchio1994). Other antagonistic behaviours, such as intraspecific parasitism (McCorquodale and Owen, Reference McCorquodale and Owen1994), have not yet been described for M. sculpturalis and can be worth considering in future studies. As highlighted by inter-annual differences in female number and nesting abundance in our study, attention to high female bee density should be given to avoid spurious results (Barthell and Thorp, Reference Barthell and Thorp1995; Guédot et al., Reference Guédot, Bosch, James and Kemp2006). Moreover, the near monofloral composition of nest pollen provisions suggests potential intraspecific competition for food resources in case of limited S. japonicum availability.
We found little evidence of parasitism in the nests inspected (only two out of 148 brood cells were parasitised), suggesting different hypotheses. First, the nest structure, with the long antechamber and the resin cover, could prevent the entrance of the common parasites of native Megachilidae species (Krombein, Reference Krombein1967; Michener and Brooks, Reference Michener, Brooks, Melo and Alves-dos-Santos2003; Morato and Martins, Reference Morato and Martins2006). Second, because M. sculpturalis is a relatively new alien species in Europe and America, native parasites may not be able to recognise it as a suitable host, although there is evidence of parasite adaptation in our and other studies (Neff, Reference Neff2021; Straffon-Díaz et al., Reference Straffon-Díaz, Carisio, Manino, Biella and Porporato2021). Last, since the studied bee hotel was in majority occupied by M. sculpturalis, there was probably no drift of parasites from nests of native species like Osmia spp., contrarily to what happened elsewhere (Straffon-Díaz et al., Reference Straffon-Díaz, Carisio, Manino, Biella and Porporato2021).
Conclusion and management remarks
Although limited by the restricted geographical area and small sample size of the native bee species compared, the duration of our study and the large number of females and nests analysed allowed us to fill a gap in the knowledge of nesting biology and nest structure of M. sculpturalis. Our results suggest that M. sculpturalis has effective nesting behaviour to avoid large parasitisation, and highlight complete niche overlap with a species of the native solitary bee genus Anthidium. Moreover, the increasing number of females observed over the 3 years of study confirms that M. sculpturalis can easily adapt to bee hotels, unlike the other exotic resin bee found in the area, Megachile disjunctiformis (Bortolotti et al., Reference Bortolotti, Luthi, Flaminio, Bogo and Sgolastra2018), and suggests that it can reproduce and spread very rapidly, which is one of the main characteristics of invasive alien species (Traveset and Richardson, Reference Traveset and Richardson2006; Stout and Morales, Reference Stout and Morales2009). Generalist exotic bees can become central nodes in invaded pollination webs, taking over the role of native species (Aizen et al., Reference Aizen, Morales and Morales2008; Geslin et al., Reference Geslin, Gauzens, Baude, Dajoz, Fontaine, Henry, Ropars, Rollin, Thébault and Vereecken2017). This characteristic, combined with our findings highlighting potential competition with native species both for food resources and nesting sites, urges the implementation of mitigation actions to reduce the spread of M. sculpturalis in non-native areas. Because bee hotels and plants of S. japonicum seem to be favourite aggregation sites of M. sculpturalis (Geslin et al., Reference Geslin, Gachet, Deschamps-Cottin, Flacher, Ignace, Knoploch, Meineri, Robles, Ropars, Schurr and Le Féon2020, and references therein; Dubaić et al., Reference Dubaić, Plećaš, Raičević, Lanner and Ćetković2022), they could become tools for its control. Bee hotels could be further used to monitor the presence and abundance of females of this species, and individual removal of their nests can reduce their expansion. Reduction in the use of S. japonicum as an ornamental plant in cities could reduce available resources, and providing shorter cavities in bee hotels could reduce the number of brood cells completed, indirectly reducing the population size of M. sculpturalis.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485323000627
Availability of data and material
The data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.8117575.
Acknowledgement
We thank Michela Boi for helping with data management and Manuela Giovanetti for helpful suggestions. We also thank Carlo Polidori and an anonymous reviewer for constructive comments that helped improve the manuscript.
Author contributions
L. Bortolotti, G. Bogo and A. Fisogni contributed to the study conception and design. L. Bortolotti, G. Bogo and A. Iannone collected data. F. Corvucci and F.-V. Grillenzoni performed palynological analyses. G. Bogo and A. Fisogni analysed the data. The first draft of the manuscript was written by G. Bogo and L. Bortolotti, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Financial support
This work was partly supported by MASAF (Italian Ministry of Agriculture, Food Sovereignty and Forestry grants to CREA – Research Centre for Agriculture and Environment) through the project BeeNet 2019–2023 (Italian National Funds under FEASR 2014–2020).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical standards
All applicable institutional and/or national guidelines for the care and use of animals were followed.










