Introduction
Broad-spectrum insecticides are the primary active constituents in most products used to manage pest ants (Williams et al., Reference Williams, Collins and Oi2001; Hoffmann et al., Reference Hoffmann, Luque, Bellard, Holmes and Donlan2016), and therefore non-target exposure of other invertebrates to ant treatment products are of high concern for invasive ant management programs. Prior to the 1960s, when ant treatment products were predominantly liquid sprays (Williams, Reference Williams1983), non-target species would have been accidentally exposed to active constituents either directly if accidentally covered in spray, or indirectly if foraging on substrates that had been covered with spray. Since the development of solid ant treatment products, however, such accidental contact exposure to active constituents contained within products does not occur because the active is ‘locked up’ within the matrix.
Of the many potential non-target species of concern for chemical regulatory authorities, honey bees (Apis mellifera; hereafter referred to as bees) have a major focus because of their importance for agriculture. Notably, bees feed on liquid sugary substances (Blackiston, Reference Blackiston2020) and so they are unlikely to be attracted to the matrices of modern solid ant treatment products such as cracked corn, fishmeal, sands or powders. Indeed I am unaware of any registered modern granular bait that contains sugar, nor any publication of bees being attracted to any modern granular ant bait. However, multiple new baits (treatment products with the matrix being an attractive food source) containing high volumes of sugar have recently been tested for use against ants (Boser et al., Reference Boser, Hanna, Faulkner, Cory, Randall and Morrison2014; Buczkowski, Reference Buczkowski, Roper and Chin2014a, Reference Buczkowski, Roper, Chin, Mothapo and Wossler2014b; McCalla et al., Reference McCalla, Tay, Mulchandani, Choe and Hoddle2020; Cabrera et al., Reference Cabrera, Fontan, Hoffmann and Josens2021), and other taxa (Kapaldo et al., Reference Kapaldo, Carpenter and Cohnstaedt2018). The presence of sugar in these baits could potentially attract bees, and any such attractancy would likely be fatal given that these products would typically contain general insecticides, including neonictinoids, which are known to be very effective at killing bees (Alburaki et al., Reference Alburaki, Cheaib, Quesnel, Mercier, Chagnon and Derome2017; Christen et al., Reference Christen, Bachofer and Fent2017).
To determine the risk of such products to bees I present four studies conducted on Norfolk Island (29° 02′S, 167° 57′E) in the Pacific Ocean assessing bee attractancy to multiple matrices that are currently being used to make unregistered ant treatment products for multiple ant eradication programs in Australia and the USA. The four studies were conducted independently of each other and varied in design because of the specific research needs of the multiple chemical regulation permits under which the products were being used, as well as occasional urgent research needs (Hoffmann, Reference Hoffmannin press). Sequential study designs, timings and observations in particular were varied to assess a variety of conditions and scenarios to provide ample opportunity for bees to interact with the treatments, as well as to eliminate potential bias and issues that may have inadvertently contributed to prior studies not obtaining any bee attractancy results. Notably, no active constituents were incorporated into any matrices in any of the trials, hence the studies used only the bait matrices to assess attractancy and therefore potential risk, not actual non-target impacts.
Methods
Matrices
For all matrices, the sugar used was Chelsea New Zealand industrial white sugar. Round hydrogels were Magic Water Beads supplied by NFL Enterprises in the USA, and irregularly shaped Hydrogels (hereafter called irregular hydrogels) were Water$ave Floragel from Polymer Innovations in Australia. Hydrogels were prepared for use by being placed in one of the multiple sugar-water solutions and allowed to absorb the solution for 24 h. Dry sugar was simply the allocated volumes of the sugar granules.
Study 1
Areas were selected where there was open mowed grass containing flowering clover (Trifolium sp.) being attended by bees. No bee hives were within the immediate vicinity. Individual flowers were selected that were less than approximately 5 cm above the ground, and no regard was given to the presence of other flowers in the vicinity. An array containing four treatments was placed directly on the grass approximately 30 cm away from the flower and with treatments equidistant from each other (fig. 1a). The four treatments were: a round hydrogel containing 30% sugar solution; an irregularly shaped hydrogel containing 30% sugar solution, a 2 g pile of dry sugar; and a 30 g pile of dry sugar. Multiple dry sugar volumes were used to account for potential differences in visual cues for bees (i.e. the 2 g pile might have been too small to be found).
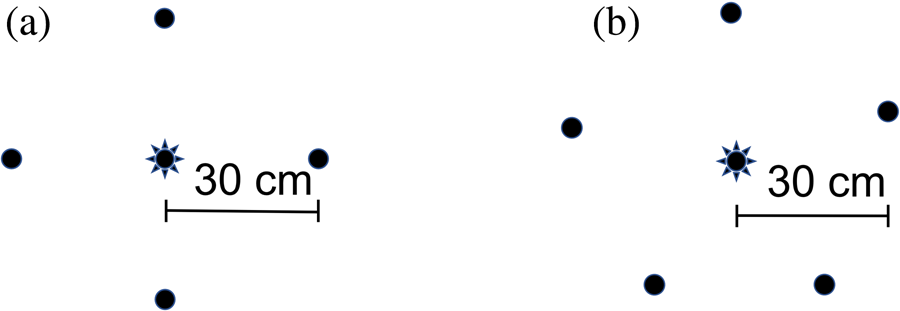
Figure 1. Arrays used in: (a) Study 1, and (b) Study 2, showing the location of treatment matrices (circles) placed around a central flower (star).
Data recorded for the flower and the four treatments per array were the number of bees that landed but did not feed, or landed and fed. Assessments were conducted for 15 min following the establishment of each array. Seventeen arrays were assessed between 27 September and 21 October 2019, all between mid-morning and mid-afternoon when temperatures ranged between 17 and 20°C and bees were visibly active.
Study 2
Forty-three arrays were assessed over a one-year period between March 2020 and March 2021 at various locations in spring, summer and autumn. Areas were selected where there was open mowed grass containing flowering clover (Trifolium sp.) being attended by bees. No bee hives were within the immediate vicinity. Individual flowers were selected that were approximately less than 5 cm above the ground and any other flowers within 70 cm of that flower were removed. To vary this study design from that of study 1, instead of the treatments being placed directly on the ground, they were placed on plates. Arrays consisted of five small (20 cm diameter) plastic plates placed exactly 30 cm from the flower and equidistant from each other (fig. 1b). About 30 ml or grams of four matrices were placed separately in four of the plates. The matrices were: hydrogels containing 30% sugar water; 30% sugar water; water; and dry sugar. The fifth plate was left empty as a control in case the plates themselves attracted bees. The position of the five treatments around the flower was always random to alleviate potential spatial bias.
Data recorded for the central flower and the five treatments around each flower were the same as in Study 1, but with the addition of ‘bee inspected quickly but did not land’ in an attempt to capture any bee interactions that may have been missed in the first study. Assessments were conducted for 10 min following the setup of each array, then after 24 and 48 h. Some fresh hydrogels were added each day half an hour prior to the 24 and 48-h assessments, and were placed in separate piles to the older hydrogels to also potentially gain insight on how hydrogel age and water loss affect attractancy and feeding.
Study 3
The third study differed from the first two studies predominantly by the arrays being positioned close to active bee hives. On 10 October 2020, five arrays were established randomly within 15 m of five sets of active bee hives around the island. Arrays consisted of four paper plates, partly filled with water to prevent interference by Argentine ant (Linepithema humile) which was present at some locations. Three rocks were placed within the water on the plates to provide a surface to place the treatments, being an irregular hydrogel containing 30% sugar water, an irregular hydrogel containing 50% sugar water, and an irregular hydrogel containing only water. These treatments were placed on three of the plates, and the rocks on the fourth plate were left empty as a control. The experiment was set up between 7:00 and 8:10am, and assessments were conducted between 8:30am and 3:50pm. Each array was monitored for 15 min approximately every 1.5 h, giving four assessments within the day. Data recorded were the same as in Study 2.
Study 4
The fourth study differed from the prior three by ensuring that all bees flying to/from hives were flying over the arrays. Twelve stations were created consisting of a foil tray with four bowls containing water, sugar water (30%), hydrogels containing 30% sugar water, and dry sugar. The bowls were placed within about 1 cm of water and did not touch the sides of the foil tray to prevent interference by ants. The spatial arrangement of the four matrices within each tray was always random to alleviate potential spatial biases. The stations were set in two arrays of six, with the two arrays set transversely to six active bee hives at distances of 10 and 50 m from the hives (fig. 2). Notably this area was directly underneath the path of bees flying to/from the hives. The arrays were established on 1 March 2021 and the trial was operated for seven days, during which time inspection of the matrices at each station was assessed twice per day at different times per day, such that by the end of the experiment inspections had been conducted at each hour from 6am to 6pm. Fresh hydrogels were added each day, but in separate piles to the older hydrogels to also potentially gain insight on how hydrogel age affects attractancy and feeding. Inspections were conducted for one minute at each station at each assessment time. Data recorded were the same as in Study 2.

Figure 2. Arrays containing multiple matrices set at 10 m from six active bee hives for Study 4 (a) and the four matrices within an array (b).
Analysis
Study 1 was analysed using a Kruskal–Wallis ANOVA, and Study 2 using a non-parametric Mann–Whitney U-test because data for both studies failed Cochran's test. No statistical analyses were conducted for Studies 3 and 4 because the data for all treatments were all zeros.
Results
Study 1
Bees were only recorded at the central flower, predominantly feeding (fig. 3; 26 out of 30 instances). No bees conducted a visual assessment of any of the matrices, none landed and none fed. These differences between bee activities at the flower vs all bee activities at all the matrices combined were statistically significantly different (Kruskal–Wallis ANOVA; H = 13.57, P = 0.0011).
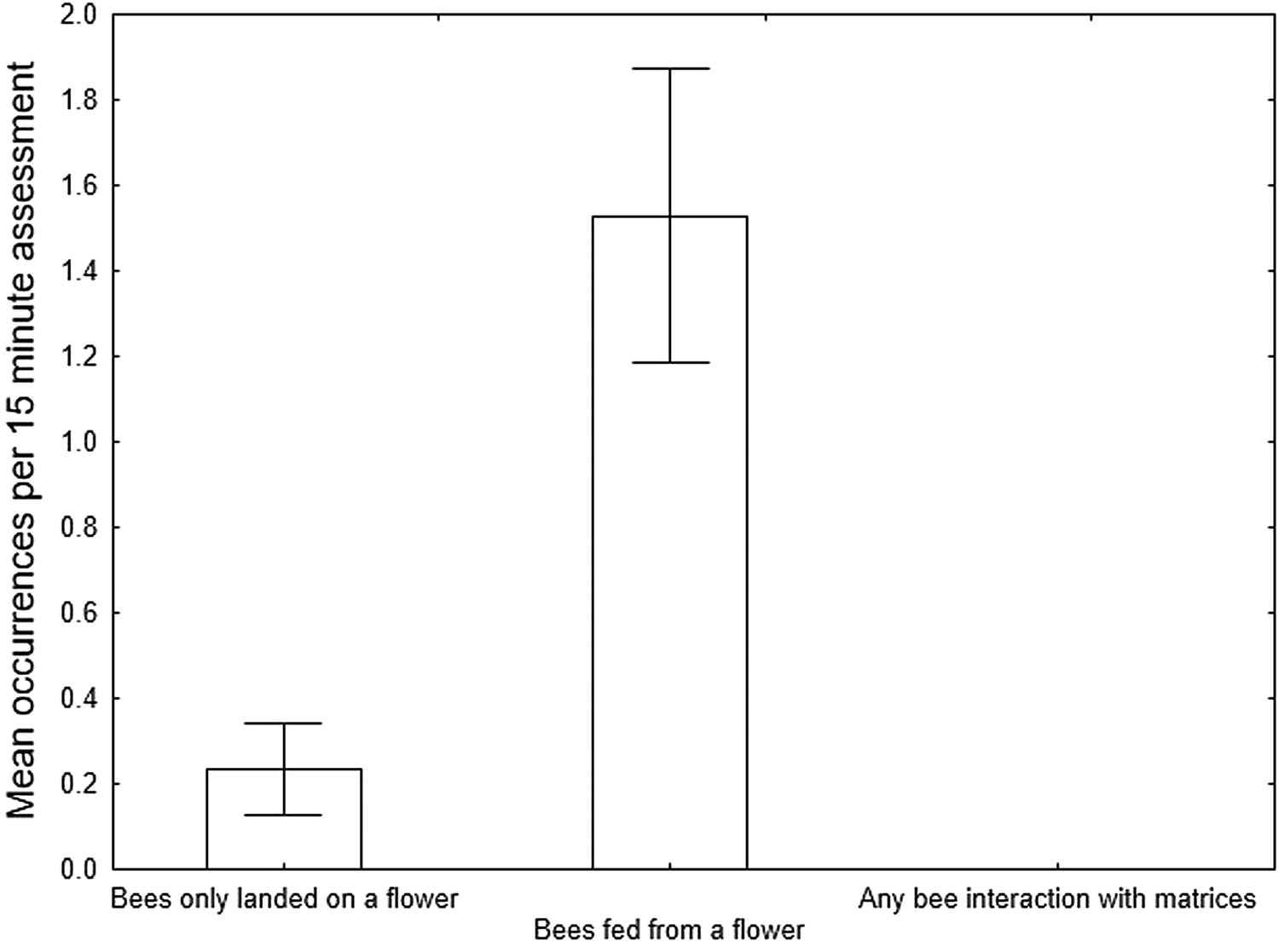
Figure 3. Mean ± SE occurrences of bee behaviour at a flower and at all treatment matrices combined in Study 1 (n = 17).
Study 2
No bees were seen inspecting or feeding on any of the matrices at any time, but bees were recorded feeding from the central flower 213 times, giving an average of 1.7 ± 0.12 (SE) flower-feeding events per 10-min assessment. Bee visitation at the central flower vs at all the matrices combined was statistically significantly different (Mann–Whitney U-test; U = 3870, Z = 7.43, P < 0.0001).
Studies 3 and 4
No bees were seen inspecting or feeding on any of the matrices at any time over the entire day in Study 3 or over the seven days in Study 4.
Discussion
These trials were conducted across multiple years, using multiple matrices containing various concentrations of sugar and in multiple forms, using various experimental setups, in different seasons, in varying locations, with many different bee hives, and with multiple observers. Not a single bee was found attracted to granular sugar or any matrix containing sugar, no bees were observed inspecting any matrix, and no bees were found feeding on any matrix, irrespective of whether the treatments were placed very near to hives and directly under bee flight paths, or out in areas where bees were feeding. This is in stark contrast to the large number of bees feeding on flowers within the immediate vicinity of all of the matrices in the first two experiments, or flying over the arrays in experiments 3 and 4 travelling to and from other food sources. Yet it is standard practice for managed bee hives to be supplementary fed with sugar (Johansson and Johansson, Reference Johansson and Johansson1977; Goodwin, Reference Goodwin1997), and bees are documented as being attracted to sugary substances (Abou-Shaara, Reference Abou-Shaara2017). I present five suggestions for the discrepancy between the trials presented here and the general perception that bees are attracted to sugar.
First, bees can indeed be attracted to some human-associated sugar sources, but these circumstances are not as simplistic as the mere presence of sugar, and predominantly they are exceptional, to the point that they are actually of science interest for their novelty as well as interesting to the popular media. Two such high-profile examples involve honeycomb discolouring. In one instance honeycomb in Brooklyn USA was stained red because bees were feeding on liquids from a maraschino cherry factory (Dominus, Reference Dominus2010). In the second instance bee hives in France were contaminated with residue from confectionary (M&M) production (National Geographic, 2012). Notably, for both incidents there was absolutely no evidence presented that bees were attracted to sugar; that inference was purely speculative. What was unique about these incidents, however, was the presence of colourings. It is just as plausible that the colourings attracted the bees, irrespective of whether the substances contained sugar or not, in much the same way that bees use floral signals associated with the light spectrum to find food sources (Schaefer et al., Reference Schaefer, Schaefer and Levey2004; Rering et al., Reference Rering, Franco, Yeater and Mallinger2020). But clearly from the experiments presented here, the light spectral properties of white sugar alone don't attract bees. In another incident, Stratford et al. (Reference Stratford, Bond, James, Roberts and Steels2002) reported ‘bee and wasp attraction to human-associated sugar’ after finding a single dead wasp beside the external tap of a sugar-syrup storage tank and observing several other wasps in the area. I personally don't consider these observations to be evidence of significant attractancy. Alternatively, however, the documented accidental and continuing mass death of bees attracted to residues of coffee, tea and juices in used paper cups in India (Chandrasekaran et al., Reference Chandrasekaran, Nagendran, Krishnankutty, Pandiaraja, Saravanan, Kamaladhasana and Kamalakannan2011) does appear to be good evidence, even though sugar was not demonstrated as being the causal attractant. Note though that in all of the instances detailed above the sugar was in a liquid or syrup form, not solid, and such instances are also seemingly rare.
Probably the greatest evidence that bees are not typically or universally attracted to human-associated sugar sources is the work of Penick et al. (Reference Penick, Crofton, Appler, Franks, Dunn and Tarpy2016) who compared stable isotope signatures of bees from managed and wild hives in urban and rural areas. Specifically, human-produced sugars are sourced primarily from sugarcane and corn syrup which have a higher δ13C than floral and insect-derived sugars. Rather than detecting higher δ13C in urban bees, which would have indicated that urban bees are utilizing human-produced sugars, the work found bees from managed hives in both areas had higher δ13C than bees from wild hives in both areas. This outcome is instead indicative of supplemental sugar feeding by beekeepers, and that urban bees surrounded by human-produced sugar sources are only feeding on natural sugar sources.
A second source of confusion could be literature involving the supplemental feeding of bee hives with sugar (fondant or pollen patties), especially in winter or during emergency situations when honey supplies get low (Johansson and Johansson, Reference Johansson and Johansson1977), or to increase pollen uptake (Goodwin, Reference Goodwin1997). But of course physically placing sugar within hives is not a demonstration of ‘attractancy’. It is instead proof that if you put sugar within the confined environment of a bee hive, especially in times when bees are starving, they will consume sugar.
A third source of confusion could be that many studies do involve honey bees feeding on sugary solutions (e.g. Oldroyd et al., Reference Oldroyd, Rinderer and Buco1991; Mangan and Moreno Reference Mangan and Moreno2009; Abou-Shaara Reference Abou-Shaara2017), but there are three problems with these scenarios. First is that the bees are trained to find the resources prior to the commencement of the study. Indeed, literature searches for this paper found no such manipulative research where bees find the resource incidentally. Second, the bees are usually starved first, or used when natural food sources are at their lowest and the bees are naturally starving, to encourage their utilization of the solutions made available. Third, these experiments are almost always conducted in laboratory settings, not open environments, and don't reflect the bee's natural food choices.
A fourth source of confusion could be that many bees and wasps are known to locate and use nonfloral natural sugar (Meiners et al., Reference Meiners, Griswold, Harris and Ernest2017). Indeed this observation was the basis for the work of Wille (Reference Wille1962) who demonstrated that honey-water mixture sprayed on leaves will attract native bees and hence can be used as a method to survey bee faunas. This knowledge has also been the basis for experiments assessing bee attractancy to sucrose solutions sprayed onto flowering crops, some of which did find increased bee activity, but others found reduced activity, with the reasoning for the vastly inconsistent results remaining unclear (reviewed in Goodwin, Reference Goodwin1997).
Bees can be attracted to sugar solutions, but this attractancy is not absolute, and the conditions for the attractancy appear to be highly nuanced. The greatest reasoning for the nuancing may indeed be due to a fifth source of confusion, in that all sugars and sugar solutions do not have equal attractancy to bees. The experiments presented here involved solely sucrose, and in multiple forms, but with no attractancy or even slight interest found, even though bees can indeed feed from hydrogels (Krushelnycky, Reference Krushelnycky2021) and have been seen taking dry sugar granules (Simpson, Reference Simpson1964). Yet bees will readily attend solutions of nectar, honeydew, and honey (Abou-Shaara, Reference Abou-Shaara2017), all of which are complexes of sugars, especially fructose and glucose, as well as amino acids, fragrance and flavour compounds among many others (Wilkins et al., Reference Wilkins, Lu and Tan1995 and references therein). The great attractancy of bees to these sugary substances very likely has led to a general and incorrect perception that bees are attracted to all sugars and sugary substances.
Two other comprehensive studies have also recently been conducted investigating pollinating-insect attractancy and feeding from various hydrogels containing sugar. On mainland USA, Buczkowski (Reference Buczkowski2020) found that honeybees and solitary bees rarely visited hydrogels and only when the hydrogels were positioned above the ground on platforms, never when hydrogels were on the ground. In one specific experiment at an apiary, bees were recorded less than 15 times, mostly during the first four hours of observations. In Hawai'i, Krushelnycky (Reference Krushelnycky2021) found that honey bees fed from hydrogels containing a sugar solution when the hydrogels were placed immediately adjacent to flowers, but not when the hydrogels were placed randomly on the ground, which suggests that the bees were not ‘attracted’ to hydrogels, but will indeed feed from them if they inadvertently encounter them. The results of these studies concur with the results of experiments presented here, as well as the broader bee literature.
Finally, as a broad observation, throughout all of the ant management work conducted on Norfolk Island over the past five years using numerous products in many locations throughout almost all times of the year, and despite constant vigilance to observe any bee interactions, no bees have ever been observed attracted to the products. Notably, no bees have been observed being attracted to the 500 l tubs containing hydrogels that sit in a single location for many weeks at a time, nor to the many locations that have been used continuously as staging areas for aerial hydrogel distribution. Even when products have been dispersed around active commercial bee hives, there has not been a single sighting of a bee showing interest in a product, and no observations of bees feeding on a product, even when weather conditions were very dry, flowers were very limited, and bees were likely hungry. The same lack of observations of attractancy has held true at the other mainland Australian locations where the hydrogels have been used extensively for ant management in Townsville (Queensland) and NE Arnhem Land (Northern Territory) (B. Hoffmann personal observations), any throughout the world where hydrogels have been used in field conditions (Boser et al., Reference Boser, Hanna, Faulkner, Cory, Randall and Morrison2014; Buczkowski et al., Reference Buczkowski, Roper, Chin, Mothapo and Wossler2014b; Peck et al., Reference Peck, Banko, Donmoyer, Scheiner, Karmi and Kropidlowski2017; Cooper et al., Reference Cooper, Hobbs, Boser and Varela2019).
In summary, this work and other assessments of bee attractancy to hydrogels and other sugar-based ant baits have found that bees are not ‘attracted’ to hydrogels, dry sugar or even sugar water. Therefore, these matrices appear to be acceptable as a basis to make treatment products for broadscale use within ant management programs. However, it should always be recognized that bees, and other non-target species, are indeed capable of feeding on these bait matrices. Therefore vigilance should still be maintained to identify special circumstances where bees may be killed when constituents are added to these matrices that do attract bees, or usage methods can adversely affect bees.
Acknowledgements
These studies were conducted at the request of the Australian chemical regulator, the Australian Pesticides and Veterinary Medicines Authority as part of permit conditions for the testing and use of experimental ant baits being used in invasive ant eradication programs, specifically permits 88159, 82931, 84820 and 84817. I thank Cath McCoy, Ben Nobbs, Greg Quinn and Lilli King for their bee assessment efforts. Thanks also to Andrea Smith for assisting with literature searches. Comments from Paul Krushelnycky, Grzegorz Buczkowski, Laura Brewington and two anonymous referees improved the draft manuscript.






