CVD is a major cause of death worldwide, accounting for 31 % of deaths globally in 2015( 1 ). As well as non-preventable risk factors such as age and ethnicity, factors such as dyslipidaemia, hypertension, abdominal obesity and pre-existing type 2 diabetes mellitus (T2DM) can contribute to the risk of CVD( Reference Pearson 2 , Reference Yusuf, Hawken and Ounpuu 3 ). Diet can play a role in managing and reducing the risk of these associated factors, and a diet high in whole grains is consistently found to be protective against CVD( Reference He, van Dam and Rimm 4 , Reference Jensen, Koh-Banerjee and Hu 5 ). ‘Whole grains’ refer to grains that contain the relative proportions of bran, germ and endosperm as they would occur naturally in the intact grain; as opposed to refined grains that have the bran and germ removed during processing. The precise mechanisms behind the cardio-protective effects of whole grains are not yet completely understood; however, many of those proposed involve the role of the cereal fibre located primarily within the bran component. For example, whole grains high in soluble fibre, such as oats and barley, may reduce the risk of CVD by decreasing serum LDL-cholesterol and improving blood glucose and insulin responses( Reference Theuwissen and Mensink 6 ).
While it is certain that the cereal fibre plays a role in the cardio-protective association of whole grain foods, there is still debate as to what extent it is responsible. There are other constituents of the whole grain that may contribute to lower risk of CVD, including Mg( Reference Fardet 7 ), vitamin E and bioactive compounds such as phyto-oestrogens( Reference Cassidy and Hooper 8 ). It is commonly considered that the contribution of these additional constituents, or perhaps, a synergistic effect of consuming the grain’s nutrients and bioactive compounds together, is greater than any benefits of cereal fibre alone, if it were consumed outside of the whole grain.
As the bran component of the grain, removed within the refining process, contains the overwhelming majority of cereal fibre as well as these other constituents within the aleurone layer, comparing whole grain with refined grain intake is not appropriate to address this question. Instead, research needs to compare or adjust whole grain intake with cereal fibre intake and bran specifically.
However, a major issue in attempts to investigate comparative association of cereal fibre, whole grain and bran to date, is the lack of consistency in definition and calculation of whole grains. Many of the large meta-analyses providing evidence for the health association of whole grains draw conclusions based on studies that define whole grains in various ways, including those that consider added bran and germ as whole grain sources( Reference Aune, Keum and Giovannucci 9 , Reference Ye, Chacko and Chou 10 ). In addition, many studies determined whole grain intake based on categorisation of mixed foods as either a whole grain or a refined grain food rather than calculating whole grain content as a dry weight of the ingredients within all foods. This method has the potential to overestimate the contribution to whole grain intake from some foods categorically considered whole grain, for example, dark breads that may use some refined flours. Alternative whole grain food definitions such as breakfast cereals that contain at least 25 % whole grain or bran( Reference Jacobs, Meyer and Kushi 11 ), or the recently developed 30 % cut-off recommended by the HEALTHGRAIN forum( Reference Ross, van der Kamp and King 12 ), have value in food labelling but are problematic in defining precise levels of intake in research. These methods can fail to capture the whole grain consumed in small amounts from food which do not meet these definitions but which could potentially accumulate to a significant contribution to total intake.
Therefore, to investigate the true cardio-protective association of whole grains, we considered the extent and quality of evidence for whole grains analysed only when using a recognised definition (namely, containing the bran, germ and endosperm in their natural proportions) and calculated on a g/100 g dry-weight basis. To provide insight into the comparative association of the cereal fibre component outside of the whole grain, we summarised evidence from studies that compared CVD-related health associations of intake of whole grain with intake of cereal fibre and/or bran.
Therefore, the aim of this review was to shed light on the relative contribution of the cereal fibre and bran components to the cardiovascular protective association found with whole grain intake.
Methods
The systematic literature review was completed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines( Reference Liberati, Altman and Tetzlaff 13 ). The details of the review were recorded and registered with PROSPERO (International Prospective Register of Systematic Reviews, http://www.crd.york.ac.uk/PROSPERO), registration number CRD42017069226 before commencement.
Inclusion and exclusion criteria
Articles were required to have considered both whole grain intake and cereal fibre and/or bran intake in association with CVD-related health outcomes in humans. Since there were only limited articles identified as meeting this criterion, no restrictions were placed on the CVD-related health outcomes examined and included. These could include but were not limited to disease biomarkers, anthropometric measures, morbidity and mortality. Results were restricted to those published in English and contained within peer-reviewed journals, and there were no restrictions based on the time of publication. Editorials, reviews, meta-analyses and conference abstracts were excluded.
Exposure – initial analysis
Articles must have reported on total whole grain intake, and those examining intake of only a defined set of whole grain foods or dietary patterns including whole grains were excluded. The definition of whole grains used was in accordance with that of American Association of Cereal Chemists International (AACCI): ‘the intact, ground, cracked or flaked caryopsis, whose principal anatomical components – the starchy endosperm, germ and bran – are present in the same relative proportions as they exist in the intact caryopsis’( 14 ). This definition is similar to those used in Europe and Australasia but critically defines whole grains, not whole grain foods. Also, it means that articles that included added bran or germ as a source of whole grain were excluded from the initial analysis.
Exposure – expanded analysis
Initial scoping found a limited amount of studies meeting the described inclusion criteria. Consequently, a second review of the evidence was conducted, expanding the criteria to include studies with a broader definition of whole grains. This expanded analysis includes studies that may not have defined whole grains as per the AACCI definition but may have utilised whole grain food definitions. For example, several articles that utilised a categorisation system of whole grain and refined grain foods, which categorised added bran and germ as a whole grain food, were included in an expanded analysis. Similarly, articles that met all other selection criteria but did not clearly explain how whole grain intake was defined or categorised were included in this expanded analysis.
Comparison
To be considered for inclusion, studies must have made a comparison of whole grain intake with cereal fibre and/or bran intake. This may have been completed through assessment of cereal fibre/bran intake separately, adjustment of whole grain intake for cereal fibre/bran or by matching whole grain and refined grain intake based on cereal fibre/bran content. Bran was included as an alternative comparison as it is the portion of the grain containing the majority of the cereal fibre.
Study design
Due to wide disparity and potential differences between acute exposure and long-term exposure, this paper reports only the results of observational studies. Intervention studies will be reported in a separate review. A summary of the participants, exposure, comparisons, outcomes and study design is presented in Table 1.
Table 1 PICOS criteria for inclusion and exclusion of studies in initial analysisFootnote *

PICOS, population, intervention, comparator, outcome, study design; AACCI, American Association of Cereal Chemists International.
* PICOS criteria, population, intervention (exposure), comparator, outcomes, study design.
† Expanded to include studies not meeting this definition in expanded analysis.
Search term and strategy
Relevant human journal articles were identified in medical databases Scopus, Web of Science, CINAHL plus, CENTRAL in Cochrane Library and PubMed published before March 2018 using the following key words: ‘cardiovascular’, ‘coronary’, ‘stroke’, ‘cholesterol’, ‘triglycerides’, ‘lipids’, ‘obes*’, ‘overweight’, ‘glucose’ and ‘diabetes’ in combination with ‘wholegrain*’ or ‘grain*’, as well as ‘cereal*’ or ‘bran’ and ‘fibre’ or ‘fiber’. The full search strategy (SCOPUS) has been presented in online Supplementary material.
The titles of the articles were screened for inclusion by one author. The abstracts of remaining articles were then reviewed. After exclusion based on abstract, the full text of each potentially eligible article was retrieved. Two authors (E. M. B. and E. J. B.) separately assessed the full text studies for inclusion in the review. The reference lists of included articles were hand searched for additional studies that met the criteria.
Data extraction
One author (E. M. B.) extracted the required information and summarised the data in table format, which was reviewed by a second author (E. J. B.). Studies meeting the accepted definition of whole grains (initial analysis) were summarised separately for expanded analysis. Within both groups, whole grain, cereal fibre and bran intake data were extracted in separate tables. Each summary table included first author’s last name, publication year, study name, participants, country in which the study was performed, CVD-related outcomes examined, highest and lowest median intake, covariate adjustments, main results and statistical significance. When bran intake data were extracted, the table also included whether the bran was total bran (naturally occurring plus added) or added bran.
Quality assessment
The design of each included study was identified and recorded. The National Health and Medical Research Council (NHMRC) levels of evidence guidelines( 15 ) were used to assign a level of evidence to each study based on design. In addition, the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies( 16 ) was used to guide critical appraisal of articles. The NIH quality assessment tool provides a checklist of fourteen criteria that can be applied to cohort and cross-sectional studies to evaluate internal validity. Studies were assessed on the basis of research objective, study population specification, participation rate, inclusion/exclusion criteria, sample size justification, time frame, exposure and outcome measurement, blinding, attrition and confounders. The NIH tool then includes a subjective quality rating (good, fair or poor) that the reviewer decides on based on their overall evaluation, rather than a direct numerical ranking.
Method of analysis
Due to the wide range of CVD-related outcomes assessed within limited studies, a meta-analysis was not possible. Instead, studies were grouped based on comparison measure (cereal fibre or bran intake) and where possible, on health outcome assessed, and data were examined and synthesised qualitatively. The separation of articles within the initial and expanded analysis allows an examination of how the quality of evidence differs when restricted to articles using the currently accepted definition of whole grains. The quality assessment process was also used to inform the synthesis of results, with studies of higher quality guiding the interpretation of findings and subsequent discussion.
Results
Our search strategy identified 1909 articles (Fig. 1). Duplicates were removed and titles were screened for relevance, with 671 articles selected for screening of abstract. After screening for both title and abstracts, ninety-six full-text citations remained, with a further seventy-three articles excluded because they did not analyse both whole grain and cereal fibre or bran intake. One article was excluded as it measured only non-CVD-related outcomes, and two articles were excluded because they defined cereal fibre as the fibre sourced from breakfast cereals only. A further twelve articles were excluded from the initial analysis because they did not use the AACCI definition of whole grain and one article excluded based on no clear description of how whole grain foods were defined (later included within the expanded analysis). Seven articles (based on three studies) remained in the initial analysis.
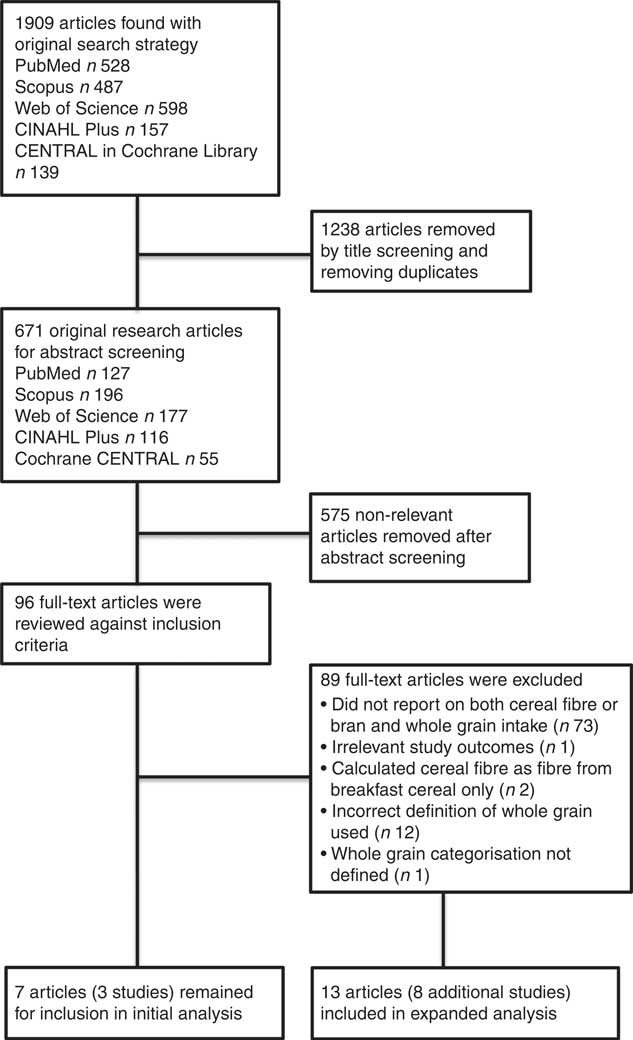
Fig. 1 Flow diagram of study selection process.
When the expanded criterion was applied to include the articles that did not use the AACCI definition of whole grain, thirteen previously excluded articles (eight additional studies) were included (Fig. 1). They were previously excluded as eleven of these articles included added bran and/or germ as a source of whole grain, one article did not include whole grain bread as a source of whole grain intake, and one article did not provide sufficient detail as to how whole grain intake was defined.
A summary of articles exploring the associations between whole grain, cereal fibre and bran intake within the initial analysis are presented in Tables 2, 3 and 4, respectively. Similarly, Tables 5, 6 and 7 present a summary of articles in the same format for when the criteria were expanded.
Table 2 Characteristics of studies within initial analysis exploring association of whole grain intake with outcomes related to CVD

PCS, prospective cohort study; NHS, Nurses’ Health Study; F, female; T2DM, type 2 diabetes mellitus; MI, myocardial infarction; RR, relative risk; HPFS, Health Professionals Follow-up Study; M, male; HR, hazard ratio; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule 1; TNF-R2, TNF receptor 2.
* Pooled HR for whole grain intake without added bran/germ. Located within Wu et al.( Reference Wu, Flint and Qi 18 ) online Supplementary Table S4.
Table 3 Characteristics of studies within initial analysis exploring associations of cereal fibre intake with outcomes related to CVD

PCS, prospective cohort study; NHS, Nurses’ Health Study; F, female; T2DM, type 2 diabetes mellitus; MI, myocardial infarction; RR, relative risk; CRP, C-reactive protein; N/A, not available; TNF-R2, TNF receptor 2; HPFS, Health Professionals Follow-up Study; M, male.
Table 4 Characteristics of studies within initial analysis exploring associations of bran intake with outcomes related to CVD

PCS, prospective cohort study; NHS, Nurses’ Health Study; F, female; T2DM, type 2 diabetes mellitus; MI, myocardial infarction; RR, relative risk; N/A, not available; HPFS, Health Professionals Follow-up Study; M, male; HR, hazard ratio; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule 1; TNF-R2, TNF receptor 2.
* Bran consumed naturally within whole grains and bran added to food/consumed separately.
† Bran added to food/consumed separately only.
‡ Located within Wu et al.(18) online Supplementary Table S7.
§ Located within Wu et al.(18) online Supplementary Table S6.
Table 5 Characteristics of studies within expanded analysis exploring associations of whole grain intake with outcomes related to CVD

HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; M, male; F, female; CRP, C-reactive protein; PCS, prospective cohort study; MI, myocardial infarction; RR, relative risk; HR, hazard ratio; T2DM, type 2 diabetes mellitus; FMC, Finnish Mobile Clinic Health Examination Survey; IWHS, Iowa Women’s Health Study; FOS, Framingham Offspring Study; HOMA-IR, homoeostatic model assessment of insulin resistance; ERA, Estrogen Replacement and Atherosclerosis trial; BLSA, Baltimore Longitudinal Study of Aging; BP, blood pressure; NLCS, Netherlands Cohort Study; N/A, not available.
* One whole grain serve (s): one slice bread; one cup breakfast cereal, rice, pasta, other grain, popcorn, cooked oatmeal; one tablespoon bran, wheat germ.
Table 6 Characteristics of studies within expanded analysis exploring associations of cereal fibre intake with outcomes related to CVD

PCS, prospective cohort study; FMC, Finnish Mobile Clinic Health Examination Survey; F, female; M, male; T2DM, type 2 diabetes mellitus; RR, relative risk; IWHS, Iowa Women’s Health Study; FOS, Framingham Offspring Study; HOMA-IR, homoeostatic model assessment of insulin resistance; HR, hazard ratio; ERA, Estrogen Replacement and Atherosclerosis trial; BLSA, Baltimore Longitudinal Study of Aging; BP, blood pressure; NLCS, Netherlands Cohort Study; N/A, not available.
Table 7 Characteristics of studies within expanded analysis exploring associations of bran intake with outcomes related to CVD

HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; M, male; F, female; CRP, C-reactive protein; PCS, prospective cohort study; RR, relative risk; HR, hazard ratio.
* Bran consumed naturally within whole grains and bran added to food/consumed separately.
† Bran added to food/consumed separately only.
‡ One serve(s) of bran=one tablespoon.
Data extraction
Within the initial analysis, seven articles, which were based on three primary studies, investigated various associations between whole grain, bran and cereal fibre intake and cardiovascular-related outcomes. The primary studies were the Nurses’ Health Study (NHS) I and II and the Health Professionals Follow-up Study (HPFS).
The following CVD-related outcomes were assessed in relation to whole grain and cereal fibre and/or bran intake within the initial analysis, each within single article: incidence of T2DM, CVD mortality and all-cause mortality in healthy participants and those with diabetes, hypertension, CHD, long-term weight gain and various inflammatory markers (Tables 2–4).
Within the expanded analysis (thirteen articles reporting on eight new studies), two articles directly compared outcomes between whole grain intake and bran intake and eight articles directly compared outcomes between whole grain intake and cereal fibre intake. One article adjusted whole grain intake for cereal fibre intake, and one article compared association between whole grain and non-whole grain intake matched on cereal fibre content. The expanded analysis investigated the incidence of T2DM (three articles), CVD mortality and all-cause mortality in healthy participants (two articles), incidence of CHD (one article), the metabolic syndrome (one article) and stroke (one article): and markers of CVD risk, anthropometric measures (three articles), blood lipid profile (two articles), blood glucose measures (three articles), inflammatory markers (one article) and change in coronary artery diameter and percentage stenosis (one article) (Tables 5–7).
Quality assessment
The level of evidence among the articles according to the NHMRC guidelines ranged from level II (twelve prospective cohort studies observing mortality or morbidity end points) to level IV (six cross sectional analysis studies) (Table 8). Five of the seven articles within the initial analysis were assigned a level II, while seven of the thirteen articles within the expanded analysis were assigned a level II.
Table 8 Classification of study design as per National Health and Medical Research Council (NHMRC) level of evidence guidelines and classification of quality as per National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies

While the majority of articles (10/19) were of good quality as determined using the NIH quality assessment tool, the ratio of good to fair quality was better in the initial analysis (6:1 v. 4:9 in the expanded analysis) (Table 8). The study design used often limited the quality that could be attained, with cross-sectional design unable to measure exposure before outcome and unable to assess associations over a sufficient time frame. In addition, articles that only assessed exposure once over the study period and articles that relied on self-reported outcome measures were often assessed as lower quality than those that assessed exposure multiple times and used validated measures.
Initial analysis
Of the seven articles that assessed whole grain intake in the initial analysis, six found a significant inverse association with CVD-related outcomes( Reference Jensen, Koh-Banerjee and Hu 5 , Reference Qi, van Dam and Liu 17 – Reference Koh-Banerjee, Franz and Sampson 21 ), however, two of these found the association attenuated when adjusting for cereal fibre and whole grain constituents( Reference Jensen, Koh-Banerjee and Hu 5 , Reference Flint, Hu and Glynn 20 ). All six articles investigating bran intake found a significant inverse association with CVD-related outcomes( Reference He, van Dam and Rimm 4 , Reference Jensen, Koh-Banerjee and Hu 5 , Reference Qi, van Dam and Liu 17 – Reference Koh-Banerjee, Franz and Sampson 21 ), although one of these articles investigated two separate cohorts and found a non-significant inverse association in one cohort( Reference de Munter, Hu and Spiegelman 19 ). There were mixed results on the association with cereal fibre, with two articles showing a significant inverse association( Reference Qi, van Dam and Liu 17 , Reference Koh-Banerjee, Franz and Sampson 21 ) between intake and risk factors, and a third article showing no association( Reference He, van Dam and Rimm 4 ).
Whole grain intake compared with bran intake
The highest intake of whole grain was associated with 12 % lower risk of CVD mortality (hazard ratio (HR) 0·88, 95 % CI 0·82, 0·96, P=0·001) and an 8 % lower risk of all-cause mortality (HR 0·92, 95 % CI 0·89, 0·96, P<0·001) compared with the lowest intake in a large combined cohort of the NHS I and the HPFS( Reference Wu, Flint and Qi 18 ). Within the same cohort, the association of high bran intake with lower risk of CVD mortality was slightly greater. A 20 % lower risk for the highest intake of total bran (HR 0·80, 95 % CI 0·73, 0·87, P<0·001) and a 24 % lower risk for the highest intake of added bran (HR 0·76, 95 % CI 0·70, 0·83, P<0·001) compared with lowest intake were observed. Similarly, high whole grain and total bran intake were both associated with lower risk of T2DM among healthy participants within the NHS I (relative risk (RR) 0·75, 95 % CI 0·68, 0·83, P<0·001 and RR 0·72, 95 % CI 0·65, 0·80, P<0·001, respectively) and the NHS II (RR 0·86, 95 % CI 0·72, 1·02, P=0·03 and RR 0·84, 95 % CI 0·71, 1·00, respectively)( Reference de Munter, Hu and Spiegelman 19 ); however, the inverse association with total bran intake within the NHS II only reached borderline significance (P=0·07).
Whole grain intake was associated with a 19 % lower risk of hypertension( Reference Flint, Hu and Glynn 20 ) (RR 0·81, 95 % CI 0·75, 0·87, P<0·0001) and a 16 % lower risk of CHD( Reference Jensen, Koh-Banerjee and Hu 5 ) (HR 0·84, 95 % CI 0·71, 0·98, P=0·02) in the HPFS, when comparing the highest quartile of intake to lowest. However, after adjusting for cereal fibre intake( Reference Flint, Hu and Glynn 20 ) and whole grain constituents including dietary fibre( Reference Jensen, Koh-Banerjee and Hu 5 ), both of these association were attenuated (P=0·23 and P=0·06, respectively), suggesting some significance of the cereal fibre component towards the whole grain association. Bran intake was also inversely associated with risk of both hypertension and CHD (RR 0·85, 95 % CI 0·78, 0·92, P=0·002 and HR 0·72, 95 % CI 0·61, 0·84, P<0·001, respectively), and the association with lower risk of CHD remained significant after adjusting for constituents (P<0·001).
Whole grain intake compared with bran and cereal fibre intake
Within the HPFS, whole grain, bran and cereal fibre intake were associated with better weight management over time( Reference Koh-Banerjee, Franz and Sampson 21 ). The association was stronger for whole grain intake than for cereal fibre or bran intake. For every 40 g/d increase in whole grain intake, 8-year weight gain was 1·1 kg lower (P<0·0001), while for every 20 g/d increase in cereal fibre or bran intake, 8-year weight gain was 0·81 kg (P=0·0002) and 0·36 kg lower (P=0·01), respectively, indicating a slightly stronger association for whole grain intake.
Lower risks of CVD mortality and all-cause mortality were found for high whole grain, cereal fibre and bran (total and added) intake in a cohort of 7822 women with existing T2DM( Reference He, van Dam and Rimm 4 ). However, after adjustment for lifestyle and dietary factors, only the associations for total and added bran intake remained significant for both outcomes. A 64 % lower risk of CVD mortality (RR 0·36, 95 % CI 0·24, 0·54, P<0·001) and a 55 % lower risk of all-cause mortality (RR 0·45, 95 % CI 0·36, 0·57, P<0·001) were observed for those consuming the highest intake of added bran compared with non-consumers.
Whole grain, bran and cereal fibre were significantly inversely related to C-reactive protein (CRP) and TNF receptor 2 levels in a fair quality analysis of women with T2DM within the NHS I( Reference Qi, van Dam and Liu 17 ), but no association was found with E-selectin or intercellular adhesion molecule 1 levels. Notably, within this study no adjustment was made for other dietary factors that may be correlated with whole grain intake and influence inflammation, such as PUFA intake.
Expanded analysis
Eleven of thirteen articles within the expanded analysis found a significant inverse association between whole grain intake and at least one CVD-related outcome analysed( Reference Jensen, Koh-Banerjee and Sampson 22 , Reference Liu, Stampfer and Hu 23 , Reference Fung, Hu and Pereira 25 – Reference Huang, Xu and Lee 29 , Reference Erkkila, Herrington and Mozaffarian 31 – Reference van de Vijver, van den Bosch and van den Brandt 34 ), though six of these found the association no longer significant after adjustment for cereal fibre intake( Reference Liu, Stampfer and Hu 23 , Reference Fung, Hu and Pereira 25 – Reference Huang, Xu and Lee 29 ), indicating cereal fibre as a key component in the association. All eight articles that analysed cereal fibre intake separately found a significant inverse association with at least one CVD-related outcome( Reference Montonen, Knekt and Jarvinen 26 – Reference Huang, Xu and Lee 29 , Reference Erkkila, Herrington and Mozaffarian 31 – Reference van de Vijver, van den Bosch and van den Brandt 34 ). One article investigating bran intake found mixed association( Reference Jensen, Koh-Banerjee and Sampson 22 ), while the other two found significant inverse association( Reference Liu, Stampfer and Hu 23 , Reference Juan, Liu and Willett 24 ).
Whole grain intake compared with bran intake
One fair quality analysis( Reference Jensen, Koh-Banerjee and Sampson 22 ) considered association of whole grain and bran intake with lipid profile, markers of glycaemic control and inflammation. The highest consumers of whole grains (quintile 5; Q5) had 2·9 % lower total cholesterol (P=0·02), 5·3 % lower HDL-cholesterol (P=0·05) and 2·1 % lower LDL-cholesterol than the lowest consumers (quintile 1; Q1), although the association with LDL-cholesterol did not reach significance (P=0·10). Highest whole grain intake (Q5) was also associated with 17·4 % lower levels of homocysteine as well as lower concentrations of C-peptide (–13·6 %, P=0·03) and leptin (–11·0 %, P=0·03). Other markers examined, including HbA1c, were not associated with whole grain intake. Similar associations were seen when examining bran intake separately but were only borderline statistically significant, with the exception of homocysteine concentrations (–10·9 %, P=0·02). Leptin was not associated with bran intake (–2·0 %, P=0·88).
Whole grain and bran intake were both associated with lower incidence of CHD in a good quality prospective cohort study( Reference Liu, Stampfer and Hu 23 ) (RR 0·75, 95 % CI 0·59, 0·95, P=0·01 and RR 0·63, 95 % CI 0·42, 0·95, P=0·001, respectively), with participants consuming one serving of added bran per d observing a 37 % lower risk of CHD compared with non-consumers. These significant associations were found after adjustment for several related lifestyle and diet covariates including smoking status, physical activity and fatty acid composition of diet, however, the association with whole grain intake was attenuated after further adjustment for grain constituents including dietary fibre (P=0·07). High total bran intake was associated with 11 % lower risk of stroke (HR 0·89, 95 % CI 0·79, 1·0, P=0·004) in a separate good quality pooled cohort study, whereas whole grain intake showed no association( Reference Juan, Liu and Willett 24 ).
Whole grain intake compared with cereal fibre intake
Whole grain intake was associated with a 21 % (95 % CI 0·65, 0·96, P=0·0089) to 35 % (95 % CI 0·36, 1·18, P=0·02) lower risk of T2DM comparing the highest intake to the lowest intake in three studies (one good quality, two fair quality)( Reference Fung, Hu and Pereira 25 – Reference Meyer, Kushi and Jacobs 27 ). Importantly, the two fair quality studies failed to adjust for potentially important covariates including physical activity( Reference Montonen, Knekt and Jarvinen 26 ) and related dietary factors such as fruit and vegetable intake( Reference Meyer, Kushi and Jacobs 27 ). A fourth study of fair quality found a 33 % lower risk (95 % CI 0·48, 0·91, P=0·01) of the metabolic syndrome( Reference McKeown, Meigs and Liu 28 ). The association completely disappeared when adjusting for cereal fibre in all four cases. The three fair quality studies also observed association for cereal fibre intake separately and found that the highest intake was associated with a 36 % (95 % CI 0·53, 0·79, P=0·0001) to 61 % (95 % CI 0·20, 0·77, P=0·01) lower risk of T2DM, and a 38 % (95 % CI 0·45, 0·86, P=0·002) lower risk of the metabolic syndrome. Meyer et al. ( Reference Meyer, Kushi and Jacobs 27 ) adjusted this result for whole grain intake, and the lower risk was only slightly reduced to 34 % (95 % CI 0·53, 0·83, P=0·0001).
The association of whole grain and cereal fibre intake with all-cause mortality and CVD-mortality was explored in a large, good quality analysis of 367 442 US participants( Reference Huang, Xu and Lee 29 ). High whole grain intake was associated with a 17 % lower risk of both outcomes (95 % CI 0·81, 0·86, P<0·0001 and 95 % CI 0·78, 0·88, P<0·001, respectively). Once adjusted for cereal fibre, the association with all-cause mortality was attenuated to 6 % (95 % CI 0·90, 0·97, P=0·002), and the association with CVD mortality completely disappeared (HR 0·95, 95 % CI 0·88, 1·03, P=0·188). High cereal fibre intake, analysed separately within the same study, was associated with very similar lower risk of all-cause and CVD mortality. In a smaller, fair quality study( Reference Jacobs, Pereira and Meyer 30 ), whole grain consumers tended to have lower risk of all-cause mortality (HR 0·83, 95 % CI 0·73, 0·94, P<0·05) compared with refined grain consumers.
The association of whole grain and cereal fibre intake to progression of coronary atherosclerosis among 229 women were analysed within a fair quality study( Reference Erkkila, Herrington and Mozaffarian 31 ). Women consuming above the median intake of whole grains (>6 serves/week) and cereal fibre intake (>3 g/4184kJ (1000kcal)) had a significantly smaller change in minimum coronary artery diameter over the 3-year period compared with those consuming under the median (–0·06 v. –0·10 mm, P=0·04 and –0·04 v. –0·09 mm, P=0·03, respectively).
Two fair quality cross-sectional articles examined whole grain and cereal fibre intake in relation to glycaemic control. In the Framingham Offspring Study, mean homoeostasis model assessment-estimated insulin resistance (HOMA-IR) was borderline significantly lower in the highest quintile of whole grain intake (quintile 5; Q5) compared with the lowest (quintile 1; Q1) (Q1 6·8 v. Q5 6·6, P=0·05); however, this association disappeared once adjusted for cereal fibre( Reference McKeown, Meigs and Liu 28 ). In contrast, the association between high cereal fibre intake and lower mean HOMA-IR remained after adjusting for whole grain intake (Q1 6·9 v. Q5 6·5, P=0·003). Within the Baltimore Longitudinal Study of Aging, higher whole grain and cereal fibre intake were similarly associated with lower postprandial glucose (quintile 1 (Q1) 8·24 mmol/l v. quintile 5 (Q5) 7·23 mmol/l, P=0·006 and Q1 8·05 mmol/l v. Q5 6·48 mmol/l, P=0·02, respectively); however, no association with fasting glucose, fasting insulin or postprandial insulin were found( Reference Newby, Maras and Bakun 32 ).
Within the same study, the highest consumers of whole grain and cereal fibre had significantly lower total cholesterol than the lowest consumers (Q1 5·79 mmol/l v. Q5 5·49 mmol/l, P=0·02 and Q1 5·73 mmol/l v. Q5 5·44 mmol/l, P=0·005, respectively). The highest consumers of both intake also had lower LDL-cholesterol than the lowest consumers, though this was borderline significant for cereal fibre intake (Q1 3·13 mmol/l v. Q5 2·9 mmol/l, P=0·07), and somewhat negatively, the highest consumers of whole grain intake had lower HDL-cholesterol, though not significantly (Q1 1·27 mmol/l v. Q5 1·22 mmol/l, P=0·07).
Participants consuming the highest intake of whole grain and cereal fibre had significantly lower anthropometric body measurements compared with those consuming the lowest intake in three cross-sectional analyses( Reference Newby, Maras and Bakun 32 – Reference van de Vijver, van den Bosch and van den Brandt 34 ), though notably two of the three studies did not perform an adjustment for physical activity within their multivariate models( Reference Newby, Maras and Bakun 32 , Reference van de Vijver, van den Bosch and van den Brandt 34 ). Within the Netherlands Cohort Study, it was estimated that every 1 g/d higher intake of whole grain was associated with a 0·03 and 0·04 kg/m2 lower BMI in men and women, respectively (P<0·01 for both)( Reference van de Vijver, van den Bosch and van den Brandt 34 ). This meant that a decrease of 1 kg/m2 BMI corresponded to a 33 and 25 g increase in whole grain intake. Every 1 g of cereal fibre intake was similarly associated with a 0·04 kg/m2 lower BMI in men (P<0·01), but no association was found for women. Within the same cohort, a high intake of whole grain was associated with a slightly lower risk of being overweight and obese in both men (OR 0·98, 95 % CI 0·96, 0·99, P<0·01 and OR 0·90, 95 % CI 0·84, 0·98, P<0·05, respectively) and women (OR 0·98, 95 % CI 0·96, 0·99, P<0·01 and OR 0·96, 95 % CI 0·93, 0·99, P<0·05, respectively), while a high intake of cereal fibre was only associated with a lower risk of being overweight in men (OR 0·98, 95 % CI 0·96, 0·99, P<0·01). van de Vijver et al. also conducted a prospective analysis of a smaller cohort of 1257 participants, with follow-up ranging 1–5 years, but no association was found between baseline intake and anthropometric measures.
Discussion
Within the review, the association between whole grains, bran and cereal fibre and lower risk of CVD-related outcomes were evident. In the expanded analysis, cereal fibre and whole grain (including added bran) intake were similarly associated with lower risk of CVD mortality, and the fibre component tended to explain the association( Reference Huang, Xu and Lee 29 ), whereas fibre-rich bran was strongly associated with lower risk of stroke incidence( Reference Juan, Liu and Willett 24 ). The inverse association between the risk of CVD-related outcomes and cereal fibre intake may be due to favourable effects on factors likely to influence the development of the disease such as serum lipids( Reference Jenkins, Kendall and Axelsen 35 ), glucose and insulin metabolism( Reference Juntunen, Laaksonen and Poutanen 36 ), blood pressure( Reference Appel, Moore and Obarzanek 37 ) and inflammation( Reference King, Egan and Geesey 38 ). These factors may also play a role in slowing progression of CVD, as higher intake of cereal fibre and whole grain products were both associated with less progression of coronary atherosclerosis in postmenopausal women with established CHD( Reference Erkkila, Herrington and Mozaffarian 31 ). The differences in progression (0·04 mm less progression in high whole grain consumers; 0·05 mm less progression in high cereal fibre consumers) were somewhat comparable with treatment effects reported in statin trials (0·06–0·08 mm)( Reference Ballantyne 39 ).
Interestingly when studies assessed added bran intake (bran added to food during processing), some of the strongest inverse association with CVD-related outcomes were found. As added bran, as opposed to total bran, does not include bran intake consumed as part of whole grain foods, there is less chance of it being simply a surrogate marker for whole grain intake, providing a more precise insight into the benefits of bran alone. In the study by He et al. ( Reference He, van Dam and Rimm 4 ) of women with T2DM, only added bran was significantly associated with lower CVD-related and all-cause mortality after adjustment for related lifestyle and dietary factors. While this study was underpowered to detect modest associations; and therefore, the non-significant associations found for whole grain and cereal fibre may be due to limited power, the same strong inverse association with CVD mortality and all-cause-mortality was found for added bran intake in healthy participants( Reference Wu, Flint and Qi 18 ). Furthermore, added bran had the strongest inverse association with CHD( Reference Jensen, Koh-Banerjee and Hu 5 , Reference Liu, Stampfer and Hu 23 ). One possible explanation for the consistently strong inverse associations found for added bran is that within these populations, bran is sourced mainly from only two sources, wheat bran and oat bran( Reference Jensen, Koh-Banerjee and Hu 5 ). Oat bran is one of the highest sources of the soluble fibre β-glucan, which is consistently associated with reduced serum total LDL-cholesterol in clinical trials( Reference Anderson, Gilinsky and Deakins 40 , Reference Davy, Davy and Ho 41 ).
However, the direct association within the cross-sectional studies of whole grain, cereal fibre and total bran intake to serum cholesterol levels were less consistent( Reference Jensen, Koh-Banerjee and Sampson 22 , Reference Newby, Maras and Bakun 32 ). Again, association may have been stronger if specifically analysing intake of high-soluble fibre grains. Cross-sectional studies are inherently unable to capture the impact of long-term trends in intake, and sample sizes were relatively small within both studies. For example, while a 4·7 % lower LDL-cholesterol level, as found in one study comparing highest consumers of cereal fibre with lowest consumers( Reference Newby, Maras and Bakun 32 ), may appear clinically relevant to reduced CVD risk( 42 ), it did not quite reach statistical significance (P=0·07).
It is thought that whole grains may contribute to weight loss through the effect of fibre on satiation and satiety( Reference Koh-Banerjee and Rimm 43 ), leading to lower energy intake. In addition, the secretion of particular gut hormones such as cholecystokinin may contribute to satiety or alter glucose homoeostasis( Reference Pereira and Ludwig 44 ), and soluble fibre may improve insulin sensitivity by slowing the absorption of macronutrients, indirectly affecting body weight( Reference Koh-Banerjee and Rimm 43 ). While the inverse association within the HPFS between whole grain, added bran and cereal fibre intake and weight gain over an 8-year period were statistically significant, the weight change differences between the highest and lowest intake were modest( Reference Koh-Banerjee, Franz and Sampson 21 ). Considering the findings for whole grains and fibre intake effect on weight regulation in clinical trials tend to be inconsistent( Reference Brownlee, Moore and Chatfield 45 – Reference Melanson, Angelopoulos and Nguyen 47 ), further research investigating this relationship is needed.
Within this review, whole grain intake tended to be associated with lower incidence of T2DM and the metabolic syndrome, but this association consistently disappeared after adjustments for cereal fibre intake. The one study that did not perform adjustments for cereal fibre intake found similar risk reductions between whole grain and bran( Reference de Munter, Hu and Spiegelman 19 ). High-soluble fibre foods have been found to reduce post-prandial glucose and insulin response( Reference Jenkins, Wolever and Leeds 48 ) and high-fibre diets may improve insulin sensitivity and lower insulin secretion( Reference Weickert, Mohlig and Schöfl 49 ). Interestingly, two studies within the review found that insoluble fibre but not soluble fibre was associated with lower risk of T2DM( Reference Montonen, Knekt and Jarvinen 26 , Reference Meyer, Kushi and Jacobs 27 ). Insoluble fibre may also slow the absorption of foods( Reference Anderson 50 ), but it is also suggested that a quicker transit time, achieved with high-insoluble fibre intake, allows less time for carbohydrates to be absorbed in the upper jejunum, thus relieving insulin demand( Reference Cummings and Englyst 51 ). Both whole grain and cereal fibre intake were associated with significantly lower HOMA-IR, a measure of insulin resistance, in the Framingham cohort even after adjustment for relevant lifestyle and dietary factors. However, the whole grain association disappeared after adjustment for cereal fibre( Reference McKeown, Meigs and Liu 28 ).
In addition to the fibre content, micronutrients within whole grains such as Mg may also play a role in insulin sensitivity and reduced risk of T2DM. In a small study of elderly participants, 4·5 g of Mg daily for 4 weeks improved glucose and insulin responses( Reference Paolisso, Sgambato and Gambardella 52 ). Within our review, the importance of Mg intake was inconclusive. Some studies found that Mg intake could not explain the whole grain association( Reference de Munter, Hu and Spiegelman 19 , Reference Montonen, Knekt and Jarvinen 26 ), while another found Mg was independently associated with reduced risk of T2DM, even after adjustment for cereal fibre( Reference Meyer, Kushi and Jacobs 27 ). Importantly, this study did not adjust for any other related dietary factors that may confound these associations. Overall, the findings suggest that the fibre component of whole grains and bran may play the major role in the inverse association with T2DM. Other nutrients such as Mg may also contribute.
CRP was the only inflammatory marker regularly assessed. Elevated CRP concentrations, a marker of inflammation, have been found to independently predict CVD in the general population( Reference Ridker, Buring and Cook 53 ) and people with T2DM( Reference Schulze, Rimm and Li 54 ), and the American Heart Association has concluded that the risk of CVD is lower when the CRP level is below 1 mg/l( Reference Pearson, Mensah and Alexander 55 ). Here, high whole grain, bran and cereal fibre intake were significantly associated with lower CRP concentrations in the same cohort of women with T2DM( Reference Qi, van Dam and Liu 17 ). Within a healthy population, high whole grain and bran intake tended to be associated with lower CRP concentrations; however, after adjustment for dietary and lifestyle factors, the association completely disappeared for whole grain and became non-significant for bran( Reference Jensen, Koh-Banerjee and Sampson 22 ). Hyperglycaemia may, in part, influence production of pro-inflammatory cytokines( Reference Gonzalez, Minium and Rote 56 , Reference Devaraj, Venugopal and Singh 57 ), so whole grain and cereal fibre intake may assist in inhibiting inflammation through an effect on blood glucose control.
The findings of this review suggest that fibre may play a significant role in the cardio-protective association of whole grains. Whole grain, bran and cereal fibre intake were associated with similarly lowered risks of most outcomes assessed, and when tested, cereal fibre often accounted considerably for whole grain associations, particularly for T2DM. When bran was separated from whole grains as in the initial analysis, intake of bran, particularly added bran, had equal or stronger inverse association with various CVD-related outcomes to that of whole grains. As bran contains the majority of cereal fibre within grains, this may further support the notion that fibre contributes considerably to the protective associations of grains. Of course, it is important to note that bran cannot be interpreted simply as a direct measure of cereal fibre, due to the abundance of minerals, phytochemicals and phyto-oestrogens contained within the aleurone layer( Reference Lillioja, Neal and Tapsell 58 ). In the HPFS cohort, adjusting for fibre and micronutrient constituents attenuated the inverse association between whole grains and CHD but did not have an appreciable effect on the strong inverse association for added bran( Reference Jensen, Koh-Banerjee and Hu 5 ). Further, Jacobs et al. ( Reference Jacobs, Pereira and Meyer 30 ) found that consumers of fibre from whole grain sources (which included bran) compared with fibre from refined grain sources tended to have lower risk of all-cause mortality but not specifically CVD mortality. We cannot disregard the possibility that these bioactive compounds within the bran aleurone layer, botanically linked to fibre, may contribute to the inverse associations found( Reference Jacobs, Pereira and Meyer 30 ).
Another important consideration in examining associations between whole grains and CVD-related outcomes is the diet and lifestyle of whole grain consumers, beyond whole grain consumption. Whole grain intake may be associated with an overall healthier lifestyle and better diet quality( Reference Kyrø, Skeie and Dragsted 59 ), and some of these associated factors such as higher physical activity levels, lower incidence of smoking and higher fruit and vegetable intake may confound or contribute to any associations attributed to whole grain intake. Within the initial analysis, all seven of the good quality articles included careful adjustment for multiple lifestyle and dietary factors in their multivariate models. However, the fair quality article( Reference Qi, van Dam and Liu 17 ) observing associations with markers of inflammation included lifestyle factors within the multivariate model, without inclusion of potentially relevant dietary factors such as polyunsaturated fat intake( Reference Kris-Etherton, Harris and Appel 60 ). Similarly, several of the articles included within the expanded analysis failed to adjust for factors such as physical activity status( Reference Montonen, Knekt and Jarvinen 26 , Reference Erkkila, Herrington and Mozaffarian 31 , Reference Newby, Maras and Bakun 32 , Reference van de Vijver, van den Bosch and van den Brandt 34 ), alcohol intake( Reference Montonen, Knekt and Jarvinen 26 ) and other dietary factors( Reference Meyer, Kushi and Jacobs 27 ). In some cases, articles reporting on the same study cohorts adjusted for considerably different factors within their multivariate models. As causation cannot be determined from observational studies, it is crucial that careful adjustment is made for all potentially confounding factors. The inconsistency among several articles within the review, particularly within the expanded analysis, may limit the quality of the overall evidence.
Many of the articles within this review also went on to adjust for constituents within the whole grain, including cereal fibre. It was interesting that associations found within the initial analysis were less prone to attenuation after adjustment for cereal fibre than in the expanded analysis. Using an accepted definition of whole grain (including all fractions in natural proportions), and methodical calculation of whole grain, bran and cereal fibre intake, results are less likely to be confounded by imprecise categorisation. We can therefore be more confident that the abundance of nutrients expected to be found within whole grains and the separate bran component were accurately captured in estimated intake. Beyond the incorrect inclusion of added bran, it is possible that the presence of non-whole grain (and therefore potentially nutrient poor) grain intake incorrectly captured as whole grain intake within the expanded analysis diluted the overall quality of intake and therefore blunted the contribution of any whole grain nutrients beyond cereal fibre. The noticeable difference in results between initial and expanded analysis suggests that while it is evident that cereal fibre is an important component of whole grain and bran, we cannot disregard contribution from nutrients beyond this.
The inconsistency in definitions and categorisation systems used, and therefore limited amount of evidence based on the correct definition of whole grains, is a considerable limitation of this review. Only three major study cohorts (the NHS I and II and the HPFS) were analysed using the correct definition of whole grains. In addition, while the wide variety of CVD-related outcomes assessed shed light on the various ways whole grain intake may be protective, we were limited in our ability to draw sound conclusions about any one risk factor or outcome, as most outcomes were assessed in few studies, in diverse populations, or using different definitions of whole grain, making a more in-depth study of results by way of a meta-analysis not possible.
Conclusion
Intake of whole grain, cereal fibre and bran (total and added) appeared to be similarly associated with lower risk of various CVD-related risk factors and outcomes within this review, suggesting that the fibre component of whole grains, located primarily within the bran fraction, may play an important role in the cardio-protective associations observed with whole grain intake. Notably, however, when the accepted definition and precise categorisation of whole grain and bran intake were used, association found were stronger and less prone to attenuation after adjustment for cereal fibre, potentially indicating a role of whole grain nutrients beyond fibre.
The findings from this review are interesting in consideration of the promotion of grain foods in public health initiatives and dietetic practice. Potentially, an overly restricted focus on whole grain cereal foods may deter consumption of high-fibre non-whole grain foods such as bran-based cereals. The similar lower risks observed for added bran separate to whole grain across both analyses raise the question of whether current initiatives and health policies should include mention of whole grain and/or bran foods. Indeed, this question may also be valuable for the food industry, whereby the reformulation of cereal products containing extrinsically sourced cereal fibre or fine bran may overcome sensory barriers to whole grain foods – while still providing cardio-protective benefits. Future adequately powered studies, including intervention studies comparing the effects of whole grain intake with fibre-matched bran or added-cereal fibre intake on CVD-related risk factors and outcomes, are needed to establish the mechanisms behind the protective effects of whole grains and their components as well as the dose–response relationships, where there is currently paucity of data and lack of agreement. These studies are needed before findings can be translated into practical application. Most importantly, there is a crucial need for future research to utilise a consistent, methodical and quantified approach when calculating whole grain intake to ensure future evidence in this area is consistent and accurate and reflects the current definition of whole grain.
Acknowledgements
The authors thank Professor Chris Seal (Newcastle University) for guidance and support in the design of the review.
This research has been conducted with the support of the Australian Government Research Training Program Scholarship. No other funding has been provided.
E. M. B. and E. J. B. designed the research; E. M. B. and E. J. B. conducted research; E. M. B. and M. J. B. analysed data; E. M. B. and E. J. B. wrote the paper; S. R. reviewed synthesis of data results and reviewed manuscript; E. M. B. had primary responsibility for final content.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451900031X












