CVD as well as osteoporosis constitute major health problems in elderly individuals. There is a marked European geographical distribution of postmenopausal osteoporosis and CVD, with rates being lower in Southern European countries. Among other factors, the Mediterranean diet, rich in herbs and nuts, seems to be strongly associated with this low incidence of degenerative diseasesReference de Lorgeril and Salen1–Reference Carluccio, Siculella, Ancora, Massaro, Scoditti, Storelli, Visioli, Distante and De Caterina4.
The data on the specific dietary constituents that are involved in the health effects of the Mediterranean diet include, among others, antioxidant polyphenols, monounsaturated and polyunsaturated lipids and phyto-oestrogensReference Gebauer, Psota, Harris and Kris-Etherton5, Reference Kris-Etherton, Hecker, Bonanome, Coval, Binkoski, Hilpert, Griel and Etherton6. Walnuts (Juglans regia) are rich in substances such as ellagic acid, a known polyphenol, α-tocopherol (vitamin E), fibre, essential fatty acids, flavonoids and phenolic acidsReference Maguire, O'Sullivan, Galvin, O'Connor and O'Brien7, Reference Fukuda, Ito and Yoshida8. The high content of PUFA (linoleic and linolenic acid) in walnuts has been suggested to reduce the risk of heart disease by decreasing total and LDL-cholesterol and increasing HDL-cholesterol. This favourable lipid profile of nuts has previously been proposed as the mechanism of walnuts’ apparent anti-atherogenic effect in manReference Almario, Vonghavaravat, Wong and Kasim-Karakas9–Reference Ros, Nunez, Perez-Heras, Serra, Gilabert, Casals and Deulofeu11. On the other hand, vitamin E (α-tocopherol), a dietary antioxidant component of walnuts, appears to be the most effective in reducing the risk of CVD, exerting its effect through increasing the antioxidant defence systemReference Wu, Koga, Martin and Meydani12. Of note, ellagic acid, a polyphenol, is a major component of walnuts and it has been proposed to exert anti-atherogenic, anti-carcinogenic and antioxidative propertiesReference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13–Reference Mertens-Talcott, Talcott and Percival15 (Fig. 1).
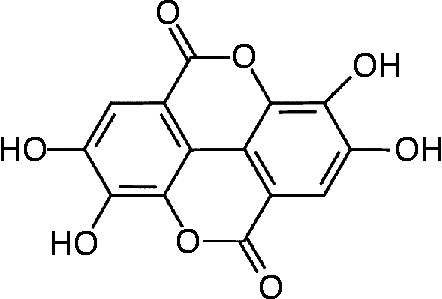
Fig. 1 Structure of ellagic acid.
The inflammatory process plays an important role in the pathogenesis of atherosclerosis through the interaction of the endothelium with the immune cells. Numerous signalling cascades have been elucidated and, among other functions, the inflammatory cytokine-induced adhesion molecules by the endothelium play a critical role in the inflammatory process and immune responseReference Han, Quon and Koh16. The adhesion molecules, namely vascular cell adhesion molecule (VCAM)-1 and intracellular cell adhesion molecule (ICAM)-1, activated by inflammatory cytokines such as TNF-α, participate in the initiation of this interaction. To evaluate the cardioprotective effect of walnut, beyond its cholesterol-lowering ability, we examined the potential of methanolic extract derived from walnuts, J. regia, as well as its major component ellagic acid, to inhibit the expression of adhesion molecules (VCAM-1 and ICAM-1), measured by cell-ELISA in human aorta endothelial cells (HAEC).
Accumulating evidence points out that many of the pathophysiological events associated with CVD are also associated with low bone densityReference McFarlane, Muniyappa, Shin, Bahtiyar and Sowers17. In addition, recent data indicate that polyphenols, flavonoids and PUFA, major components in walnuts, exhibit favourable effects on bone health either by decreasing bone resorption or by increasing osteoblastic activity and bone formationReference Park, Han, Suh, Ryu, Hyon, Cho and Park18–Reference Tokuda, Takai, Hanai, Matsushima-Nishiwaki, Hosoi, Harada, Ohta and Kozawa24. In view of the above and because ellagic acid, a known polyphenol present in abundance in walnut methanolic extractsReference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13, Reference Colaric, Veberic, Solar, Hudina and Stampar25–Reference Jakopic, Colaric, Veberic, Hudina, Solar and Stampar30, has shown a mineralisation effect on osteoblastsReference Papoutsi, Kassi, Tsiapara, Fokialakis, Chrousos and Moutsatsou31, we assessed further the effect of walnut methanolic extract in osteoblasts and compared it with that observed in the presence of ellagic acid. We used an osteoblastic cell line (KS483) that has the ability to form mineralised nodules in vitro. The differentiation and mineralisation process, assessed by Alizarin Red-S staining, is a widely accepted assay that reflects the potential of tested compounds to promote osteoblastic activity.
Experimental methods
Materials
HAEC, as well as the endothelial cell basal medium and Single Quots Bulletin kit, containing human epidermal growth factor, hydrocortisone, gentamycin, amphotericin B, bovine brain extract and fetal bovine serum (FBS), were purchased from Clonetics® (Cambrex Corporation, East Rutherford, NJ, USA); all other cell-culture materials, such as α-minimum essential medium, HEPES-buffered saline solution, trypsin–EDTA solution and Dulbecco's minimal essential medium and FBS were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). TNF-α (T0157), ( ± )-α-tocopherol (vitamin E; T3251), l-ascorbic acid (vitamin C; A4544), β-glycerophosphate (G9891), Alizarin Red-S (A5533), 17β-oestradiol (E4389), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; M5655) and the peroxidase substrate o-phenylendiamine hydrochloride (FAST™ OPD; P9187) were obtained from Sigma-Aldrich (St Louis, MO, USA). V-CAM antibody (M7106) was purchased from DakoCytomation (Glostrup, Denmark), I-CAM antibody (BBA3) was purchased from R&D Systems (Minneapolis, MN, USA) and sheep anti-mouse IgG secondary antibody (NA931) was purchased from Amersham (Little Chalfont, Bucks, UK). All other chemical materials were purchased from Sigma-Aldrich, Fluka (Buchs, Switzerland) and BioRad (Hercules, CA, USA).
Preparation of the extract and ellagic acid
A quantity of 1260 g powdered peeled dried fruits of J. regia L. were extracted at room temperature, firstly to be defatted, with cyclohexane (3 × 2500 ml), giving 387 g of a yellow oily residue, after the evaporation of the solvent. Then, the plant material was extracted with methanol (3 × 2000 ml) and, after evaporation of the solvent, an orange oily extract (412 g) was obtained. A quantity of 4 g of the methanolic extract was subjected to reverse-phase column HPLC (15 × 230 mm, Buchi, R18 silica gel 60, 20–40 mm; Merck, Darmstadt, Germany) and eluted with water followed by water–methanol mixtures (250 ml each one) of decreasing polarity, to yield eight major fractions. Fraction 5 was further purified on Sephadex LH-20 CC eluted with methanol to provide ellagic acid (25 mg). The chemical structure of ellagic acid was identified by direct comparison with an authentic sample (through TLC and NMR spectroscopy).
High-performance liquid chromatography analysis
Analytical HPLC was performed using the Thermo Finnigan HPLC system with a photodiode-array detector (Spectra System UV6000LP; Thermo Scientific, Waltham, MA, USA) and scanning wavelength range 200–380 nm. Separations were carried out using a Lichrosorb RP 18 column (250 × 4·0 mm, 5·0 μm particle size) and the system was controlled by CromQuest™ 4.0 software. The analysis was carried out with a binary mobile-phase gradient with a total flow rate of 1·0 ml/min. Solvent A consisted of 2 % acetic acid and solvent B consisted of 0·5 % acetic acid–acetronitrile (ratio 1:1, v/v). The 80 min gradient method began with 90 % A to 45 % A in 50 min, 0 % A in 10 min, 90 % A in 5 min and finally 90 % A for 15 min for equilibration between each analysis. The injection volume was 20 μl. Before analysis each sample was dissolved in 50 % aqueous methanol to 10 mg/ml concentration for the methanolic extract and 1 mg/ml concentration for the pure compounds (standards) gallic acid, catechin, caffeic acid, coumaric acid and ellagic acid, which are some of the known predominant components in walnut phenolic-enriched extracts. All the samples were passed through nylon acrodisc filters (0·45 μm; Fisher Scientific, Pittsburgh, PA, USA) before analysis. The identification of the compounds contained in the extract was achieved through the comparison of their retention times and absorption maxima in the scanned spectrum with those of standard solutions.
Determination of total polyphenol content
Dry total methanolic extract (267 mg) was extracted with 2 ml methanol. After centrifugation for the removal of insoluble material the supernatant fraction was examined for polyphenol content using the Folin–Ciocalteau reagentReference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13 using 4-methylcatechol (MC) as the standard for the preparation of the calibration curve.
Culture of endothelial cells
HAEC were cultured at 37°C in a humidified 95 % air–5 % CO2 atmosphere in endothelial cell basal medium supplemented with human epidermal growth factor (10 ng/ml), hydrocortisone (1 mg/ml), gentamycin (50 mg/ml), amphotericin B (50 ng/ml), bovine brain extract (12 μg/ml) and FBS (2 %) (called hereafter endothelial growth medium (EGM)). At 70–80 % confluence, cells were washed twice with HEPES-buffered saline solution (pH 7·2–7·5), harvested with 0·025 % trypsin–0·01 % EDTA and plated at a density 2500 cell/cm2. All experiments used HAEC of passage four to eight. For experiments, cells were grown to confluence in ninety-six-well plates using EGM. Cells were washed once with HEPES-buffered saline solution (pH 7·2–7·5) and then fresh EGM containing methanolic extract from J. regia at final concentrations of 10, 50, 200 μg/ml or ellagic acid at final concentrations of 10–7–10–5 m was added and an 18 h incubation period followed. Subsequently, TNF-α (1 ng/ml) was added and the cells were incubated for an additional 6 h period. Cells treated with TNF-α (1 ng/ml) alone and cells treated with TNF-α (1 ng/ml) plus α-tocopherol (20 μm) were also included. Control cells were incubated in EGM alone (without TNF-α or compounds). Subsequently, cells were used for the measurement of protein levels of cell adhesion molecules (VCAM-1 and ICAM-1) by cell-ELISA and for the assessment of cell viability by the MTT assay.
The preparation of α-tocopherol solution was as follows: a stock solution of α-tocopherol at 10 mg/ml was prepared in ethanol and stored at − 70°C. To prepare α-tocopherol solution for cell culture, stock solution was first mixed with FBS at a ratio of 1 : 20, then incubated at 37°C for 15 min, during which time a brief vortex was conducted every 5 min. The FBS–α-tocopherol solution was then diluted by EGM medium to make the final concentration of α-tocopherol for supplementing HAECReference Wu, Koga, Martin and Meydani12.
Measurement of cell adhesion molecules by cell-enzyme-linked immunosorbent assay
Surface expression of VCAM-1 and ICAM-1 was quantified by cell-ELISA performed on an HAEC monolayer in flat-bottomed ninety-six-well plates as described previouslyReference Zhang, Stocker, McCall, Forte and Frei32. Briefly, following incubation, the cells were fixed with 0·1 % glutaraldehyde in PBS for 30 min at 4°C. Plates were blocked at 37°C for 1 h with 5 % skimmed milk powder in PBS, and an incubation at 4°C overnight with a primary monoclonal mouse antibody anti-human ICAM-1 or VCAM-1, at final concentration 2 μg/ml in 5 % skimmed milk PBS, followed. Then the plates were washed three times with 0·1 % Tween-20 in PBS and incubated with a horseradish peroxidase-conjugated sheep anti-mouse secondary antibody at a dilution of 1:5000 at room temperature for 1.5 h. Subsequently, the plates were washed three times with 0·1 % Tween-20 in PBS and finally the expression of cell adhesion molecules was quantified by the addition of the peroxidase substrate o-phenylendiamine hydrochloride. The absorption of each well was measured at 492 nm using a microplate spectrophotometer.
Cell viability
Cell viability was assessed by morphology under a phase-contrast microscope and by reduction of the tetrazolium salt MTT by mitochondrial dehydrogenases as described previouslyReference Denizot and Lang33. Briefly, after incubation of cells under all experimental conditions used, HAEC were washed twice with PBS and the medium was replaced with MTT dissolved at a final concentration of 1 mg/ml in Dulbecco's minimal essential medium (serum-free, phenol red-free) and a further 4 h incubation followed. Then, the MTT-formazan was solubilised in isopropanol and the optical density was measured at a wavelength of 550 nm and a reference wavelength of 690 nm.
Culture of KS483 osteoblastic cell line
KS483 cells were grown in α-minimum essential medium supplemented with 10 % FBS and penicillin–streptomycin, in a CO2 incubator (5 % CO2–95 % air) at 37°C and subcultured every 3–4 d at a dilution 1 : 5 to 1 : 6 using a trypsin 0·125 %–EDTA 0·01 % solution.
Before each experiment, cells were maintained for 3–4 d in α-minimum essential medium (phenol red-free) supplemented with 10 % dextran-coated charcoal-treated FBS. For the experiments, cells were seeded in twelve-well plates, at a density of 45 000/well and cultured in α-minimum essential medium with 10 % dextran-coated charcoal-treated FBS. At 3 d after plating, cells reached confluence and were subsequently induced to differentiate by the addition to the culture medium of ascorbic acid (50 μg/ml) and in the absence or presence of walnut methanolic extract at final concentrations of 10, 25 and 50 μg/ml. 17β-Oestradiol (10− 9–10− 6 m) as well as ellagic acid (10− 9–10− 6 m) were used as positive controls. β-Glycerophosphate was added after day 10. The medium with the reagents was refreshed every 3–4 d for 24 d in total. After 24 d, the cultures in twelve-well plates were rinsed with PBS, followed by fixation with 5 % formalin for 10 min and stained for Ca deposition with Alizarin Red-S (solution 2 %; pH 5·5) for 5 min. Mineralised nodules were counted by light microscopy at a 10-fold magnification as described previously.
Statistical analysis
Data are reported as mean values and standard deviations of three independent experiments (each experiment was conducted in triplicate or quadruplicate). Data in the figures are expressed as percentage of control, which was calculated as follows: (value for cells treated with compounds/value for control cells) × 100. Statistical analysis was performed using Student's t test, two-tailed distribution, assuming two-sample unequal variance.
Results
High-performance liquid chromatography and total polyphenol content
As shown in the chromatograph (Fig. 2), in the methanolic extract of walnut can be detected, among others, in considerable proportions the followings phenolic compounds: gallic acid, catechin, caffeic acid, coumaric acid and ellagic acid. Their retention times are 3·673, 9·983, 13·458, 21·275 and 33·292 min respectively.
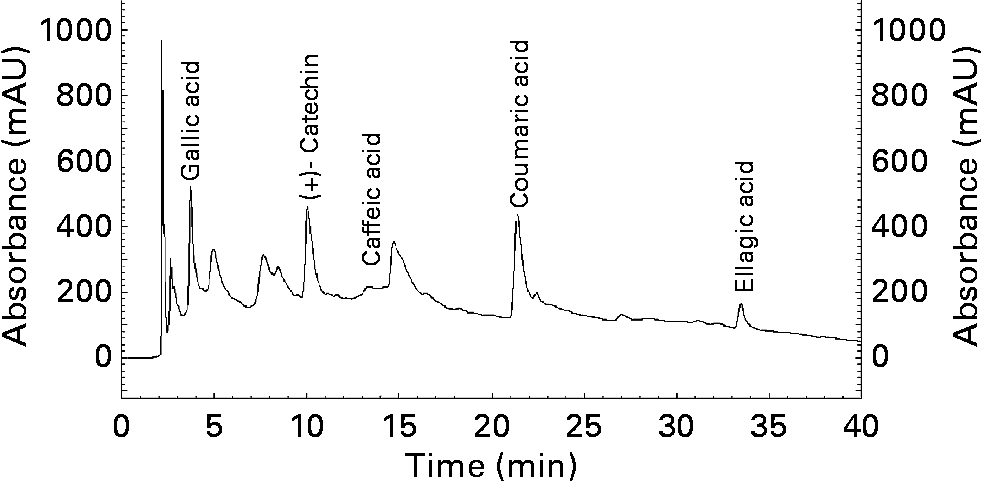
Fig. 2 HPLC chromatogram (Lichrosorb RP 18 column (250 × 4·0 mm, 5·0 μm) at 280 nm) of methanolic extract of walnuts (Juglans regia L.). mAU, milliabsorbance units.
The total polyphenol content in the dry methanolic extract was calculated to be 16·9 (sd 0·8) μmol MC/g dry substance.
Measurement of cell adhesion molecules by cell-enzyme-linked immunosorbent assay
The effect of different concentrations of TNF-α (1 or 2 ng/ml) on VCAM-1 and ICAM-1 expression was initially determined after 6, 12 or 24 h incubation. Incubation of confluent HAEC with TNF-α (1 ng/ml) caused a maximal surface expression of VCAM-1 and ICAM-1 after 6 h of incubation (data not shown). In subsequent experiments, we used TNF-α (1 ng/ml) for 6 h to induce stimulation of cells. To investigate whether the plant extract derived from J. regia inhibits TNF-α-induced protein expression of adhesion molecules, confluent HAEC were treated for 18 h in the absence or presence of extracts at concentrations of 10–200 μg/ml. Then, TNF-α (1 ng/ml) was added and cells were incubated for another 6 h period. Confluent HAEC incubated with α-tocopherol (20 μm) plus TNF-α (1 ng/ml) served as the positive control, whereas cells without TNF-α and compounds comprised the control cells.
TNF-α increased the basal expression (control) of cell adhesion molecules VCAM-1 and ICAM-1 of confluent HAEC by 201·5 (sd 15·5) and 209·9 (sd 9·4) % respectively. α-Tocopherol decreased significantly the TNF-α-induced endothelial expression of both V-CAM and I-CAM (P < 0·001), as expected. J. regia methanolic extract decreased TNF-α-induced endothelial expression of both VCAM-1 (Fig. 3 (A)) and ICAM-1 (Fig. 3 (B)) at a concentration range of 10–200 μg/ml in a statistically significant way (P < 0·01 to P < 0·001). Ellagic acid decreased TNF-α-induced endothelial expression of VCAM-1 and ICAM-1 at a concentration range of 10− 7–10− 5 m in a statistically significant way (P < 0·05 to P < 0·0010).
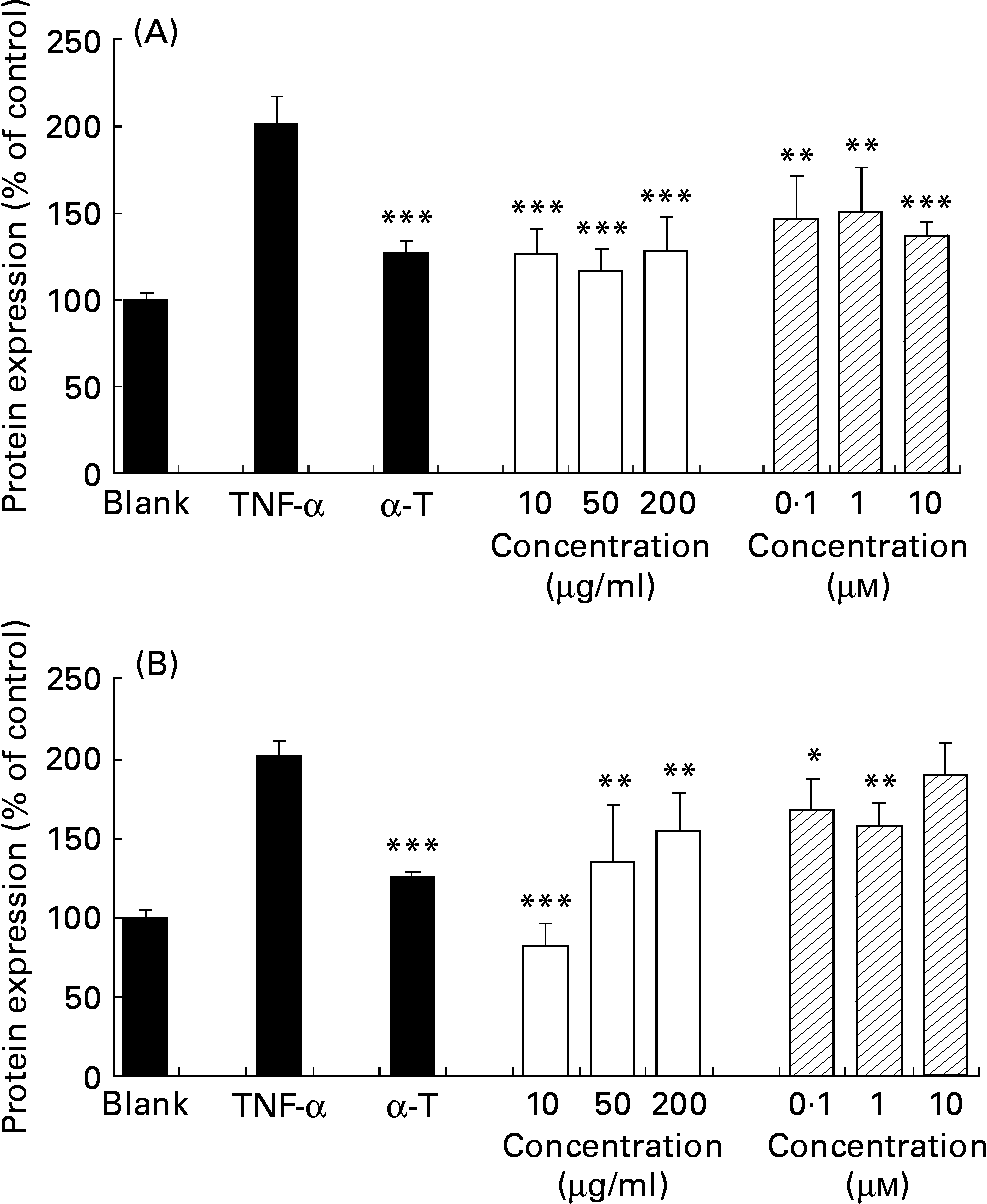
Fig. 3 Extracts inhibit TNF-α-induced vascular cell adhesion molecule-1 (A) and intracellular cell adhesion molecule-1 (B) protein expression in human aorta endothelial cells (HAEC). As described in the Materials and methods section, HAEC were incubated in the absence of TNF-α or methanolic extract (control), with α-tocopherol (α-T; 20 μm), or with different concentrations of walnut (Juglans regia L.) extract (□; 10, 50, 200 μg/ml) or ellagic acid (![]() ; 10− 7–10− 5 m) for 18 h, followed by stimulation with TNF-α (1 ng/ml) for up to 24 h. Adhesion molecules were measured by cell-ELISA. Data are expressed as percentage of control and shown as means of three independent experiments (each conducted in triplicate), with standard deviations represented by vertical bars. Mean value was significantly different from that of TNF-α-treated cells: *P < 0·05, ** P < 0·01, *** P < 0·001.
; 10− 7–10− 5 m) for 18 h, followed by stimulation with TNF-α (1 ng/ml) for up to 24 h. Adhesion molecules were measured by cell-ELISA. Data are expressed as percentage of control and shown as means of three independent experiments (each conducted in triplicate), with standard deviations represented by vertical bars. Mean value was significantly different from that of TNF-α-treated cells: *P < 0·05, ** P < 0·01, *** P < 0·001.
Cell viability
The assessment of cell viability revealed that neither the morphology nor the reduction of MTT salt in HAEC was affected by any of the compounds (plant extracts, α-tocopherol or TNF-α) at any concentration range or experimental conditions used. The lowering effect of the walnut extract and ellagic acid on the expression of adhesion molecules without affecting the proliferation rate of cells supports the anti-inflammatory activity of the compounds tested.
Mineralisation effect on KS483 osteoblasts
The bone study revealed that the methanolic extract of walnut induced nodule formation. Fig. 4 shows the effect of vehicle control (in the absence of compounds), 17β-oestradiol and walnut extract on the mineralised nodule formation. 17β-Oestradiol at a concentration range of 10− 7–10− 9 m induced significantly the nodule formation, as expected. Ellagic acid at a low concentration range (10− 8–10− 9 m) induced significantly nodule formation (P < 0·01 and P < 0·05, respectively). Treatment with the methanolic extract of J. regia stimulated the formation of mineralised nodules at concentrations of 10 and 25 μg/ml in a statistically significant way (P < 0·05 and P < 0·001, respectively).
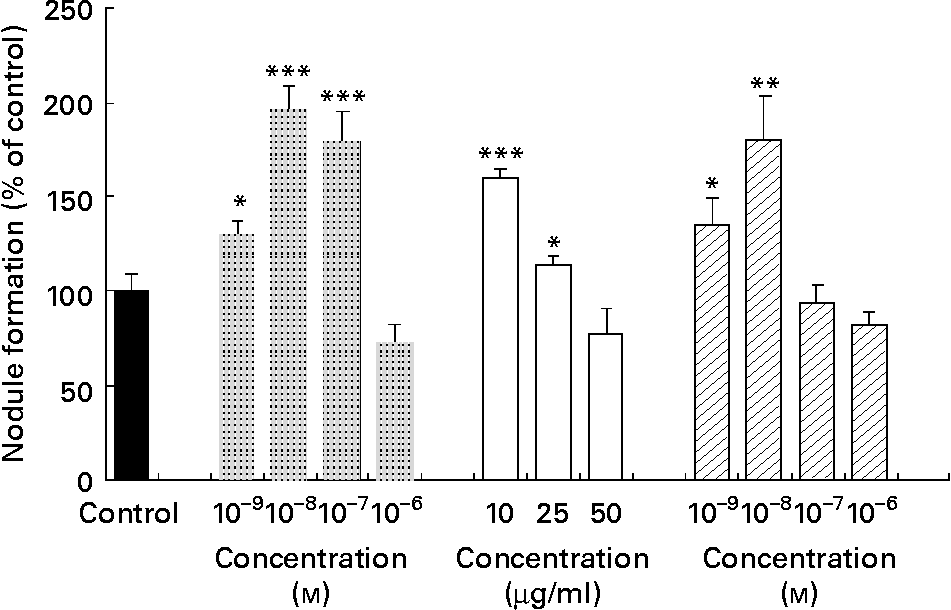
Fig. 4 Effect of 17β-oestradiol (![]() ), walnut (Juglans regia L.) extract (□) and ellagic acid (
), walnut (Juglans regia L.) extract (□) and ellagic acid (![]() ) on mineralisation of extracellular matrix in KS483 cells. Cells were exposed to vehicle control, 17β-oestradiol (10− 9–10− 6 m), methanolic walnut extract (10–50 μg/ml) and ellagic acid (10− 9–10− 6 m). Results are expressed as percentage of control (vehicle) and shown as means of the results of four cultures, with standard deviations represented by vertical bars. Mean value was significantly different from that of the vehicle control: * P < 0·05, ** P < 0·01, *** P < 0·001.
) on mineralisation of extracellular matrix in KS483 cells. Cells were exposed to vehicle control, 17β-oestradiol (10− 9–10− 6 m), methanolic walnut extract (10–50 μg/ml) and ellagic acid (10− 9–10− 6 m). Results are expressed as percentage of control (vehicle) and shown as means of the results of four cultures, with standard deviations represented by vertical bars. Mean value was significantly different from that of the vehicle control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Discussion
The cardiovascular protective effect of a walnut diet has been related to antioxidant and hypocholesterolaemic effects as well as via modulation of endothelial functionReference Almario, Vonghavaravat, Wong and Kasim-Karakas9, Reference Zambon, Sabate, Munoz, Campero, Casals, Merlos, Laguna and Ros10, Reference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13, Reference Tsuda and Nishio34. Data, however, on the anti-inflammatory effects of walnuts are sparse. Since the binding and recruitment of circulating monocytes to vascular endothelial cells are early steps in the development of inflammation and atherosclerosis, mediated through cell adhesion molecules that are expressed on the surface of endothelial cells, we evaluated the potential of the methanolic extract of J. regia, rich in polyphenolic compounds, to influence the expression of VCAM-1 and ICAM-1 by HAEC. We used the cell-ELISA to measure VCAM-1 and ICAM-1, a well-recognised in vitro assay to evaluate the anti-inflammatory effect of compounds or extractsReference Kaneko, Hayashi, Saito and Miyasaka35, Reference Wolle, Hill, Ferguson, Devall, Trivedi, Newton and Saxena36. As a positive control, we used vitamin E (α-tocopherol), an antioxidant known to exert its effect through modulation of cytokines, adhesion molecules, mobilisation of NF-κB transcription factor and interaction of immune cells with endothelial cellsReference Wu, Koga, Martin and Meydani12, Reference Weber, Erl, Pietsch, Strobel, Ziegler-Heitbrock and Weber37. In the present study, the data from the walnut methanolic extract were compared with those from ellagic acid in view of its large contribution to the total polyphenolic content. Previous reports have also demonstrated the abundance of ellagic acid in walnut extractReference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13, Reference Colaric, Veberic, Solar, Hudina and Stampar25–Reference Jakopic, Colaric, Veberic, Hudina, Solar and Stampar30. In order to decide the proper dosages of walnut methanolic extract and ellagic acid to be tested in our in vitro systems, we considered it important to take into account the following: (1) one serving of walnuts of 50 g corresponds to eight or nine shelled walnutsReference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13; (2) experimental evidence supports that 50 μm-ellagic acid is equivalent to the dietary intake of approximately 200 g blackberries (about 90 mg ellagic acid) or 350 g strawberries (about 70 mg ellagic acid)Reference Mertens-Talcott, Talcott and Percival15, Reference Walgren, Walle and Walle38–Reference Sellappan, Akoh and Krewer41; (3) a bioavailability study has shown that consumption of a pomegranate juice (180 ml) containing ellagic acid (25 mg) and ellagitannins (318 mg) resulted (after 1–3 h post-injection) in ellagic acid plasma concentrations of 0·106 and 0·06 μm respectivelyReference Seeram, Lee and Heber42; (4) our data on ellagic acid preparation indicate that 50 g of powdered peeled dried fruits of J. regia (eight or nine shelled walnuts) may result in approximately 100 mg ellagic acid; (5) the polyphenol content of walnut methanolic extract determined in the present study was 16·9 (sd 0·8) μmol MC/g dry substance, implicating that the tested walnut extract concentrations (10–200 μg/ml) correspond to concentrations of approximately 0·2–7·0 μmol MC equivalents which are within the range of expected physiological plasma levels of dietary phenolicsReference de Vries, Hollman, Meyboom, Buysman, Zock, van Staveren and Katan43–Reference Harris, Besselink, Henning, Go and Heber47. Taken together, we decided to assess the biological effects of walnut extract at a concentration range of 10–200 μg/ml and ellagic acid at a concentration range of 10− 9–10− 5 m, which are physiologically achievable and comparable concentrations. Previous studies have also shown that other polyphenolic compounds at similar concentrations, such as vitamin E (40 μm) or tea flavonoid (60 μm-epigallocatechin-3-gallate), reduce cytokine-induced adhesion molecule expression and monocyte adhesion to endothelial cellsReference Islam, Devaraj and Jialal48, Reference Ludwig, Lorenz, Grimbo, Steinle, Meiners, Bartsch, Stangl, Baumann and Stangl49. Vitamin E inhibited the TNF-α induced expression of both ICAM-1 and VCAM-1, as expectedReference Wu, Koga, Martin and Meydani12, Reference Zapolska-Downar, Zapolski-Downar, Markiewski, Ciechanowicz, Kaczmarczyk and Naruszewicz50. The methanolic extract from walnuts inhibited the TNF-α-induced endothelial activation and expression of ICAM-1 and VCAM-1 adhesion molecules, at a concentration range of 10–200 μg/ml or 0·2–7 μmol MC equivalents which are within the reported in vitro concentration range of walnut extract shown to inhibit LDL oxidationReference Anderson, Teuber, Gobeille, Cremin, Waterhouse and Steinberg13. The inhibitory effect of walnut extract was higher at low concentrations (10 μg/ml) than at higher concentrations (200 μg/ml), possibly due to the presence of multiple components present in the extract which may result in synergistic or antagonistic actions respectivelyReference Kassi, Papoutsi, Fokialakis, Messari, Mitakou and Moutsatsou51. Ellagic acid, a major component of walnut methanolic extract, inhibited also the TNF-α-induced endothelial activation and expression of ICAM-1 and VCAM-1 adhesion molecules, when tested at a concentration range of 0·1–10 μm. Yu et al. Reference Yu, Wang, Liu and Chen52 demonstrated that ellagic acid (at 25–50 μm) inhibited the IL-1β-induced endothelial activation and expression of ICAM-1 and VCAM-1 adhesion molecules. Of note, they showed that ellagic acid mediated its anti-inflammatory effects via modulation of NF-κB activityReference Yu, Wang, Liu and Chen52. The present results and those reported by Yu et al. Reference Yu, Wang, Liu and Chen52 imply that ellagic acid may confer the favourable effect of the walnut extract on endothelial function. However, this is not clearly confirmed by the present experiments since the walnut extract in the present study contained not only ellagic acid but other phenolic compounds such as gallic acid, catechin, caffeic acid and coumaric acid, which have been previously shown to inhibit endothelial activation and ICAM-1 and VCAM-1 expressionReference Ludwig, Lorenz, Grimbo, Steinle, Meiners, Bartsch, Stangl, Baumann and Stangl49, Reference Murase, Kume, Hase, Shibuya, Nishizawa, Tokimitsu and Kita53. Moreover, in vitro studies have demonstrated the ability of other compounds, also important constituents of walnuts, such as vitamins (α-tocopherol), other selected phenolics as well as PUFA, to inhibit endothelial activation and adhesion molecule expressionReference Li, Tsao, Yang, Liu, Zhu and Young26, Reference Stampar, Solar, Hudina, Veberic and Colaric29, Reference Jakopic, Colaric, Veberic, Hudina, Solar and Stampar30, Reference Ludwig, Lorenz, Grimbo, Steinle, Meiners, Bartsch, Stangl, Baumann and Stangl49, Reference Murase, Kume, Hase, Shibuya, Nishizawa, Tokimitsu and Kita53–Reference Zhao, Etherton, Martin, West, Gillies and Kris-Etherton56. Thus, ellagic acid, despite being quantitatively a predominant polyphenol in walnut extract, may not be the primary determinant that predicts the anti-inflammatory activity of walnut extract. Furthermore, it is important to mention that the ellagic acid present in walnut extract may be a result of its decomposition from ellagitannins, which are native molecules present in walnuts. Ellagitannins are polyphenols that occur as complex polymers of high molecular weight (up to 4000 kDa) and can be hydrolysed with acids or bases to yield ellagic acid, which can be used to indirectly quantify ellagitanninsReference Cerda, Tomas-Barberan and Espin57.
Summarising, our data demonstrate the anti-inflammatory potential of polyphenolic-rich walnut extract and its abundant component ellagic acid on endothelial cells, at concentrations that are considered physiologically achievable in vivo. Such data provide a further insight into the mechanism of how walnut intake may participate in cardioprotection by improving endothelial function.
Bone remodelling is characterised by a balance between osteoclast bone resorption and osteoblast bone formation. Compounds that stimulate osteoblast proliferation, differentiation, mineralisation and survival, while are suppressors of osteoclastogenic molecules, are considered to favour bone functionReference Manolagas, Kousteni and Jilka58, Reference Kousteni, Han, Chen, Almeida, Plotkin, Bellido and Manolagas59. Since the control of osteoblast differentiation and mineralisation are among the key processes in the regulation of bone mass, we decided to assess the potential of walnut extract to induce mineralisation in osteoblasts. Previous work in our laboratory has shown that ellagic acid, similarly to oestradiol, exhibited significant osteoblastic activity in the KS483 cell line from mouse calvaria, which is a non-transformed stable subclone of a parental cell line KS483 that undergoes in vitro osteoblast differentiation in association with formation of a bone-like mineralised extracellular matrix, as evaluated by Alizarin Red-S stainingReference Papoutsi, Kassi, Tsiapara, Fokialakis, Chrousos and Moutsatsou31. Based on this reliable biological test, reflective of osteoblastic activity, we evaluated the potential of the methanolic extract of J. regia to stimulate mineralisation of osteoblasts in comparison with ellagic acid. We used as a positive control 17β-oestradiol (10− 9–10− 6 m). Our findings revealed that the walnut extract stimulated the formation of mineralised nodules at a concentration range of 10–25 μg/ml, which, of note, is within the reported range at which the extract is also efficacious in preventing adhesion molecule expression by the endothelial cells. Given the high contribution of ellagic acid to the total polyphenolic content in walnuts, it can be proposed that this phenolic compound may be considered, at least in part, as a potential contributor to the apparent favourable effect of the walnut extract on the mineralisation process in KS483 osteoblasts. However, previous investigators have reported that other compounds, also important constituents of walnuts such as flavonoids, other phenolics and n-3 PUFA, regulate various processes of bone function such as differentiation, apoptosis and bone resorptionReference Griel, Kris-Etherton, Hilpert, Zhao, West and Corwin20–Reference Tokuda, Takai, Hanai, Matsushima-Nishiwaki, Hosoi, Harada, Ohta and Kozawa24. In addition, the combination of constituents present in walnut extract may result in synergistic or additive activity.
Conclusions
The methanolic extract from J. regia has a high anti-inflammatory potential that can be attributed, partly, to ellagic acid. Our data extend existing data regarding the cardioprotective effect of walnuts and provide a further molecular mechanism underlying the beneficial effect of walnuts on endothelial function. The beneficial effect of walnut extract and ellagic acid on osteoblast function suggests that the incorporation of walnuts into the diet may provide health benefits not only to the cardiovascular system, but also to the skeletal system by preventing osteoporosis.
Acknowledgements
We would like to thank Dr T. Yamashita, Head of Nephrology, Pharmaceutical Research Laboratories, Kirin Brewery Co., Ltd, Japan, and Dr M. Karperien, Department of Endocrinology and Metabolic Diseases, Leiden University Medical Center, The Netherlands, for providing us with the cell line KS483. We also thank the Bodossaki Foundation and the General Secretariat of Research and Technology, Ministry Development (EPET II) for financing the cell-culture facilities. The present study was supported by the grant EPAN TP27 from The General Secretariat of Research and Technology, Ministry of Development, in cooperation with the companies Gaea Products SA, Aktina SA, Yiotis SA, Pierre Fabre Hellas, and Attiki Bee Culturing Co. – Alexandros Pittas SA.






