Chronic gastritis, caused by Helicobacter pylori infection, is an early-stage precursor for gastric adenocarcinoma(Reference Correa1, Reference Uemura, Okamoto and Yamamoto2). However, gastric carcinogenesis may result from a combination of factors, particularly in individuals who react strongly to inflammation or demonstrate a strong immune response(Reference Sasazuki, Inoue and Sawada3). C-reactive protein (CRP) and serum amyloid component A (SAA) are acute-phase inflammatory reactants in the human body that increase in parallel(Reference Sasazuki, Inoue and Sawada3, Reference Libby4). Evidence has shown that both CRP and SAA are increased in individuals with gastritis and stomach cancer(Reference Sasazuki, Inoue and Sawada3, Reference Ilhan, Ilhan and Akbulut5, Reference Maury6). Vitamin C has been suggested to have roles in inhibiting the growth of H. pylori, inhibiting intragastric formation of nitrosamines and regulating the immune response(Reference Jarosz, Dzieniszewski and Dabrowska-Ufniarz7–Reference Jenab, Riboli and Ferrari9). Controlling the level of these biomarkers may reduce the risk of carcinogenesis in the stomach. Therefore, we hypothesise that a high serum level of ascorbic acid may reduce stomach cancer risk via control of the inflammatory markers CRP and SAA.
A population-based double-blind randomised controlled trial in a Japanese population with gastritis in an area of high stomach cancer incidence was conducted between 1995 and 2000, with the aim of examining the effect of vitamin C supplementation on the primary prevention of gastric cancer(Reference Tsubono, Okubo and Hayashi10, Reference Tsugane, Tsubono and Okubo11). We report the impact of vitamin C supplementation on CRP and SAA status in trial subjects at the end of the 5-year period.
Materials and methods
Study participants
The trial was initially intended to examine the effects of supplementation with β-carotene (0 or 15 mg/d) and vitamin C (50 or 500 mg/d) on the incidence of gastric cancer, whereby participants were randomised in a double-blind manner to one of four groups by using a 2 × 2 factorial design. A total of 1231 subjects who were aged 40–69 years and living in four municipalities of the Yokote Public Health Center District of Akita Prefecture were selected to participate in the randomised clinical trial. After the first year of participants' recruitment in 1995, β-carotene supplementation was reported to have potential harmful effects for individuals at high risk for lung cancer(Reference Omenn, Goodman and Thornquist12, 13), and the study protocol was modified by removing subjects who were using β-carotene and stopping recruitment of new subjects in three municipalities(Reference Tsubono, Okubo and Hayashi10). The primary endpoint of the trial was changed from a 10-year accumulated incidence of gastric cancer to 5-year changes of the serum levels of pepsinogen (PG) and other biomarkers(Reference Tsubono, Okubo and Hayashi10). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the ethics committee of the National Cancer Center and the Hiraka General Hospital. Written informed consents were obtained from all individuals willing to participant and those remaining in the study. Finally, 120 and 124 subjects in the low-dosage and high-dosage groups of vitamin C supplementation, respectively, completed the 5-year study (Fig. 1). The details of the study rationale, design, methodology and protocol amendment have been described previously(Reference Tsubono, Okubo and Hayashi10, Reference Tsugane, Tsubono and Okubo11).

Fig. 1 Flow chart of participant recruitment before and after the protocol amendment and of participants at the 5-year follow-up.
Eligible subjects were diagnosed with chronic atrophic gastritis by the cut-off value of PGI < 70 ng/ml and a ratio of PGI:II of < 3·0, of which the sensitivity was 80 % and specificity was 70 % as reported(Reference Miki14). Miki(Reference Miki15) reported that the values measured by the same kit showed a good correlation (correlation coefficient 0·983 for PGI, 0·991 for PGII and 0·935 for PGI:II) with those measured by RIA (PGI/PGII RIA-BEAD; Dinabot Company Limited), in which a sensitivity of 70·5 % and a specificity of 97·0 % for atrophic gastritis, compared with histology, have been reported(Reference Watanabe, Kurata and Mizuno16). Selection criteria were no history of gastric cancer or related surgery; no history of cirrhosis, liver cancer or other cancer within the last 5 years; no abnormal liver function; no use of diet supplements containing β-carotene or vitamin C; and no expectation of moving outside the study area within 1 year.
Participant follow-up and dietary intake assessment
Participants were asked to visit the community centres every 3 months where their clinical symptoms and side effects from vitamin C supplementation were assessed, compliance was checked based on the number of unconsumed capsules, and capsules for further use were dispensed(Reference Tsubono, Okubo and Hayashi10, Reference Tsugane, Tsubono and Okubo11). Compliance averaged 92·6 and 92·2 % in the low- and high-dosage groups, respectively(Reference Kim, Sasazuki and Sasaki17). A validated 138-item FFQ was used to assess dietary intake, for which participants were asked how often they consumed individual food items and to estimate the representative size of their portions relative to the size of a standard portion. Daily intake of vitamin C and other nutrients were calculated by using the fifth revised and enlarged edition of the Standard Tables of Food Composition in Japan(18). The details of the FFQ have been described in a previous report(Reference Tsugane, Tsubono and Okubo11, Reference Kim, Sasazuki and Sasaki17).
Biochemical analysis
Fasting blood samples were collected at baseline and after 5 years and analysed for serum ascorbic acid levels, CRP and SAA. The subjects were asked not to eat or drink anything except water after 21.00 hours on the day before blood sampling. The serum was sampled between 07.00 and 10.00 hours. All samples were stored at − 70 to − 85°C and were analysed simultaneously after completion of the 5-year follow-up. All assays were conducted by persons who were blinded as to the intervention assignment and the questionnaire data.
Serum for ascorbic acid measurement was stabilised by the addition of metaphosphoric acid, and serum ascorbic acid concentration was measured fluorimetrically (iodine oxidation and condensation with 1,2-phenylenediamine). CRP and SAA concentrations were determined by the latex agglutination nephelometric immunoassay test (LZ test ‘Eiken’ CRP-HG and LZ test ‘Eiken’ SAA, respectively; Eiken Kagaku Company Limited). IgG antibodies to H. pylori were measured with a direct ELISA kit (E Plate ‘Eiken’ H. pylori antibody; Eiken Kagaku Company Limited). Levels of IgG were categorised as seropositive and seronegative for H. pylori according to the selected cut-off value (492 nm)(Reference Sasazuki, Sasaki and Tsubono19).
Statistical analysis
We followed the intent-to-treat analysis, which included all subjects remaining in the study after the protocol was modified. The per-protocol analysis included subjects who completed the study to the 5-year follow-up. Baseline comparisons between the low- and high-dosage groups and the dropout group as the control were examined by one-way ANOVA for continuous variables and by the χ2 test for categorical variables. Differences of values within the low- and high-dosage groups were tested by the paired t test for continuous variables and by the one-sample z test for proportions.
CRP was categorised into positive and negative groups by using a cut-off point of 1·8 mg/l, while SAA was grouped as positive or negative based on a cut-off point of 8·0 μg/ml(Reference Sasazuki, Inoue and Sawada3). Subjects' status on combined biomarkers of CRP and SAA was determined by the defined positive and negative statuses of CRP and SAA. Log transformation was done for dietary intake of vitamin C, serum CRP and SAA, and H. pylori titre when conducting the comparisons between the two dosage groups; and data are presented as geometric means with their standard errors. The difference between the two dosage groups for changes in CRP and SAA at the end of the 5-year follow-up compared with baseline was calculated by using the geometric means, respectively.
Adjusted analysis of the means of serum CRP and SAA for covariates was performed by one-way ANOVA. Results were adjusted for age (continuous), sex, dietary intake of vitamin C (quartile), alcohol consumption (never or occasional, regular), smoking status (never, ever), BMI ( < 25, ≥ 25 kg/m2), H. pylori status (no, yes) and menopausal status (no, yes, for women). Stratified analysis was performed for age groups, alcohol consumption, smoking status, BMI and menopausal status. P values less than 0·05 in two-tailed tests were considered as significant, and all statistical analyses were performed using SAS version 9.1 (SAS Institute).
Results
The baseline characteristics of the trial participants are shown in Table 1. Subjects in the low-dosage group were older than those in the high-dosage group. There were more CRP-positive subjects in the high-dosage group than in the low-dosage group both in the intent-to-treat and per-protocol analyses (borderline significance). H. pylori titres were higher in the high-dosage group than in the low-dosage group, with a significant difference in the per-protocol analysis.
Table 1 Baseline characteristics of the participants in the trial (Mean values and standard deviations or standard errors; number of participants and percentages)
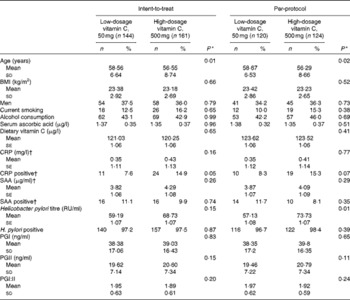
CRP, C-reactive protein; SAA, serum amyloid component A; RU, relevant unit; PG, pepsinogen.
* By one-way ANOVA test or χ2 test.
† 117 subjects in the per-protocol analysis were available in the low- and high-dosage groups, respectively.
At the 5-year follow-up, serum ascorbic acid was higher in the high-dosage group (increased 0·37 μg/l) compared with the low-dosage group (increased 0·10 μg/l from baseline, P< 0·001) (Table 2). Correlation of the log-transformed CRP and SAA in all participants at the 5-year follow-up was 0·541 (P< 0·001). A slight increase in the low-dose group and a decrease in the high-dose group both in CRP and SAA levels were observed at the 5-year follow-up; thus the absolute 0·07 mg/l reductions in CRP and the 0·31 μg/ml reduction in SAA were in the high-dose group compared with those in the low-dose group, if taking consideration of the baseline values. However, there were no significant differences for CRP between the low- and high-dosage groups (0·39 (95 % CI 0·04, 4·19) mg/l and 0·38 (95 % CI 0·03, 4·31) mg/l, respectively; P= 0·63) or for SAA between the low- and high-dosage groups (3·94 (95 % CI 1·04, 14·84) μg/ml and 3·85 (95 % CI 0·99, 14·92) μg/ml, respectively; P= 0·61) (Table 2). CRP status changed from positive to negative for 60 % (six out of ten) of the low-dosage group and 68·4 % (thirteen out of nineteen) of the high-dosage group between baseline and the 5-year follow-up (P= 0·33), while SAA status for 57·1 % (eight out of fourteen) in the low-dosage group and 70·0 % (seven out of ten) in the high-dosage group of SAA-positive participants changed from positive to negative (P= 0·27). The combined positive and negative statuses for CRP and SAA were also not significantly different between the two groups at the 5-year follow-up (47·4 % (nine out of nineteen) v. 59·1 % (thirteen out of twenty-two); P= 0·23). When we deleted two outliers that were both CRP- and SAA-positive at baseline, similar null results for CRP and SAA were observed, respectively, between the two dosage groups at the 5-year follow-up.
Table 2 Comparisons of serum ascorbic acid and inflammatory biomarkers between baseline and the 5-year follow-up (Mean values and standard deviations or standard errors)

CRP, C-reactive protein; SAA, serum amyloid component A.
* By paired t test.
† By one-way ANOVA test for the difference between the two dose groups at the 5-year follow-up.
‡ Adjusted for age, sex, BMI, smoking status, alcohol consumption, dietary vitamin C, Helicobacter pylori status and baseline level of CRP or SAA.
Stratified analysis showed that there were no significant differences in the decrease in CRP or SAA levels between the two dosage groups by age categories (40s, 50s and 60s), sex, smoking or alcohol consumption. Similar results were observed after adjusting for sex, dietary intake of vitamin C (quartile), H. pylori titre, smoking status, alcohol consumption and BMI (data not shown).
Discussion
We did not observe any significant reduction of CRP or SAA levels in the low- or high-dosage groups after 5 years of ascorbic acid supplement use, although serum ascorbic acid concentration was higher in the high-dosage group than in the low-dosage group. We also did not observe any significant differences between the two groups in age, sex, smoking, alcohol consumption or body weight status.
The CRP and SAA levels in the present study were similar to those reported in other studies(Reference Sasazuki, Inoue and Sawada3, Reference Park, Jeon and Kim20). In the present study, based on cut-off points of 1·8 mg/l for CRP and 8·0 μg/ml for SAA, there were small numbers of CRP- or SAA-positive participants and there was no significant difference for either between the two dosage groups at baseline, respectively. We also applied other cut-off points for CRP- and SAA-positive status such as a CRP of 10 mg/l(Reference Kubota, Moriyama and Yamagishi21) or by areas under the received curve(Reference Cao, Xu and Lin22). By these criteria, the numbers of CRP- or SAA-positive participants remained similar and no significant differences existed between the two dosage groups. Nevertheless, the small number of CRP- and SAA-positive participants at baseline made it difficult to evaluate changes in CRP and/or SAA status at follow-up. It might be possible that CRP and SAA were not highly sensitive markers for measuring chronic infection status, which contributed to the null outcome in the present study. On the other hand, the 500 mg/d supplement in the present study might not be sufficient to control chronic gastric infection, although cancer chemoprevention trials with more than 500 mg/d of vitamin C supplementation have not shown consistent results on the beneficial effects(Reference Mera, Fontham and Bravo23, Reference Greenberg, Baron and Tosteson24).
Human gastric carcinogenesis is a multistep and multifactorial process, with the initial stages of gastritis and atrophy linked to excessive salt intake and H. pylori infection(Reference Kim, Sasazuki and Sasaki17, Reference Correa25). H. pylori eradication can prevent the progression of precancerous gastric lesions and probably reduce the incidence of gastric cancer in those without advance lesions(Reference Fock, Talley and Moayyedi26). In the present study, CRP and SAA were not significantly reduced and the positive proportions of H. pylori were consistently higher ( ≥ 92 %) after 5 years of follow-up in both the low- and high-dosage groups(Reference Kim, Sasazuki and Sasaki17). It was possible that in the achlorhydric stomach, H. pylori infection might disappear, although the antibodies in the serum might maintain a longer time. Nevertheless, H. pylori infection potentially modulates the effects of vitamin C or vice versa(Reference Jenab, Riboli and Ferrari9). Without eradicating the infection, ascorbic acid supplementation for participants with atrophic gastritis might have fewer effects on CRP/SAA control. However, studies on changes in CRP after H. pylori eradication are contradictory. Some studies have reported a significant reduction of CRP levels in subjects after H. pylori eradication by antibiotics(Reference Kebapcilar, Bilgir and Cetinkaya27) or vitamin C supplementation(Reference Block, Jensen and Dietrich28), while others have shown no significant reduction of CRP by anti-inflammatory or antibiotic treatment(Reference Park, Jeon and Kim20, Reference Pancorbo, Vazquez and Fletcher29, Reference Eshmuratov, Nah and Kim30). A Colombian study in gastritis patients, applying a 2-week anti-H. pylori treatment and/or a 6-year antioxidant supplement, showed that acute inflammation disappeared soon after the H. pylori treatment, while chronic inflammation responded at a slower pace, and the antioxidant effect was transient and disappeared after the 6 years of follow-up, while the anti-H. pylori treatment effect persisted for as long as patients remained free of H. pylori (Reference Mera, Fontham and Bravo23). Also, subjects with non-metaplastic multifocal atrophic gastritis had the steepest declines if they cleared the bacteria, but had the sharpest increases if they did not(Reference Mera, Fontham and Bravo23). The present study results appear to support the finding that ascorbic acid supplementation does not have much beneficial effect on chronic gastric infections, particularly without assigning the anti-H. pylori treatment.
There are several limitations in the present study. The most critical one is that we did not have a placebo group for comparison with the 50 and 500 mg dosage groups(Reference Sasazuki, Hayashi and Nakachi31). However, the mean dietary intakes of vitamin C were 151·95 (sd 111·98) μg/l and 147·93 (sd 99·81) μg/l for the high- and low-dose groups, respectively, and the low-dose supplementation group was similar to or within 1 sd of the estimated vitamin C intake level from foods. In the pilot study(Reference Sasaki, Tsubono and Okubo32) for the present trial, there were no significant differences in serum vitamin C concentrations between the placebo (0 mg/d) and the low-dose groups at 1, 2 and 3 months of supplementation, respectively. Moreover, the purpose of the present study was to evaluate the effect of vitamin C supplementation (500 mg/d) compared with the normal level (the average consumption level of Japanese). Additionally, the similar mean dietary intake of vitamin C in the placebo group was seen in another trial(Reference Huang, Appel and Croft33). Therefore, the low-dose vitamin C supplementation group (50 mg/d) in the present study could be regarded as the placebo group for interpretation(Reference Kim, Sasaki and Sasazuki34). Second, the initial sample size was considered with estimated differences in accumulated gastric cancer incidence between the two study groups in 10 years rather with the changes in these biomarkers of atrophic gastritis in 5 years(Reference Tsubono, Okubo and Hayashi10). For example, to detect the 0·15 μg/ml difference in SAA levels between the two dose groups at the 5-year follow-up, using the standard deviation in each group, 5 % type I error and 20 % type II error for estimation, 1030 subjects in each group are needed. The limited number of study subjects after changes in the initial study protocol had less statistical power for identifying the significance of CRP and SAA reductions between the two dosage groups. Third, IL-6 and other immunological factors are thought to be mediators that stimulate CRP production(Reference Ilhan, Ilhan and Akbulut5, Reference Block, Jensen and Dietrich28, Reference Castell, Gomez-Lechon and David35); however, we could not evaluate the CRP reduction as modified by ascorbic acid by using these factors because the data were unavailable. Since we did not conduct endoscopy for gastritis participants, we therefore could not evaluate the progression or regression of gastric lesions after ascorbic acid supplementation at the 5-year follow-up(Reference Mera, Fontham and Bravo23, Reference Correa, Piazuelo and Camargo36, Reference Correa, Fontham and Bravo37). Finally, since we only tested CRP and SAA two times, at baseline and the 5-year follow-up, any changes in their levels in the intervening time were not evaluated.
Some studies have reported that antioxidant supplementation, even at low doses, can have adverse effects on subjects at high risk for cancer or those with undiagnosed cancer(Reference Qiao, Dawsey and Kamangar38, Reference Hercberg, Kesse-Guyot and Druesne-Pecollo39). It should be noted that some of the well-known beneficial effects of ascorbic acid administration are still only understood at the phenomenological level(Reference Mandl, Szarka and Banhegyi40). Currently, the Asia–Pacific guidelines on gastric cancer prevention do not recommend vitamin C supplementation for reducing the risk of gastric cancer(Reference Fock, Talley and Moayyedi26).
In summary, we did not observe a significant reduction in CRP or SAA levels in atrophic gastritis participants with ascorbic acid supplementation of less than 500 mg/d at the 5-year follow-up. The present study suggests that ascorbic acid supplementation might not have much beneficial effect in individuals with chronic H. pylori infection. Further studies are needed in larger populations on the control of chronic infection and inflammation through ascorbic acid supplementation.
Acknowledgements
We wish to express our appreciation to the staff at the Hiraka General Hospital and the public health nurses at Sannai village office for their support and assistance with the study. This study was supported by Management Expenses Grants from the Government to the National Cancer Center and 3rd Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan. Y. T. and S. T. designed the current research; Y. T. and S. T. initiated, modified and set the study protocols; Y. T., S. Sasaki, S. O. and S. Sasazuki implemented the study and collected the data; E. M. and S. Sasazuki analysed the data; E. M. and S. Sasazuki wrote the paper. All authors read and approved the final manuscript. The authors declare that there are no conflicts of interest.





