After the first successful hydrogenation of oils in 1897, the proportional intake of trans isomers of unsaturated fatty acids has dramatically risen in the human dietReference Emken1. Trans-fatty acid consumption is estimated to contribute 4–12 % of the total dietary fat intake in the US population, which corresponds to 13 g trans-fatty acids/person per d at the higher intakeReference Allison, Denke, Dietschy, Emken, Kris-Etherton and Nicolosi2. Unlike Western diets, traditional diets in Korea and Japan contain relatively small quantities of trans-fatty acids, with estimates in the range of 0·1–0·6 g/person per dReference Craig-Schmidt3. Trans-fatty acids occur naturally at relatively low levels in meat and dairy products as a by-product of fermentation in ruminant animalsReference Emken1. The majority of trans-fatty acids in the diet are trans-8 : 1, which is derived from the partial hydrogenation of oilsReference Lichtenstein4. However, the process of heating vegetable oils during deodorisation, and frying or baking food in vegetable oils results in the generation of trans-18 : 2Reference Kemeny, Recseg, Henon, Kovari and Zwobada5. The elevated temperature in these processes causes the conversion of cis double bonds to trans isomers.
The effect of increased trans-fatty acid consumption has been linked to a variety of afflictions, most notably CHD. Numerous epidemiological studies have correlated elevated dietary intake of trans-fatty acids with increased morbidity and mortality from CHD. Willett suggested that replacing partially hydrogenated fat with natural non-hydrogenated vegetable oils could prevent 30 000–100 000 CHD-related premature deaths each yearReference Willett6. By evaluating fatty acid intake and mortality over 25 years, the Seven Countries Study reported a correlation between trans-fatty acid consumption and the risk of death from CHD (r 0·78; P < 0·001)Reference Kromhout, Menotti and Bloemberg7. Similar findings were also reported in the Health Professionals Follow-up StudyReference Ascherio, Rimm, Giovannucci, Spiegelman, Stampfer and Willett8, the Alpha-tocopherol Beta-carotene Cancer Prevention StudyReference Pietinen, Ascherio, Korhonen, Hartman, Willett, Albanes and Virtamo9 and the Nurses' Health StudyReference Hu, Stampfer, Manson, Rimm, Colditz, Rosner, Hennekens and Willet10. A Danish study also linked trans-fatty acid consumption to the development of atherosclerosisReference Stender, Dyerberg, Holmer, Ovesen and Sandstrom11.
Compared with the consumption of an equal amount of energy from saturated or cis-unsaturated fats, the consumption of trans-fatty acids raises levels of LDL-cholesterol, reduces levels of HDL-cholesterol and increases the total cholesterol:HDL-cholesterol ratio, a powerful predictor of the risk of CHDReference Stampfer, Sacks, Salvini, Willett and Hennekens12. Although these effects would be expected to increase the risk of CHD, the relationship between the intake of trans-fats and the incidence of CHD reported in prospective studies has been greater than that predicted by changes in serum lipid levels aloneReference Ascherio, Katan, Zock, Stampfer and Willett13–Reference Mensink, Zock, Kester and Katan15, suggesting that trans-fatty acids may also influence other risk factors for CHD.
Recent studies suggest multiple possible mechanisms that might mediate the association of trans-fatty acids with CVDReference Ascherio16. For example, trans-fatty acids influence PG balance, which in turn promotes thrombogenesisReference Kinsella, Bruckner, Mai and Shimp17 and inhibits the conversion of linoleic acid to arachidonic acid and to other n-6 PUFA, perturbing essential fatty acid metabolism and causing changes in the phospholipid fatty acid composition in the aortaReference Kummerow, Zhou, Mahfouz, Smiricky, Grieshop and Schaeffer18. Trans-fatty acids have been associated with the activation of systemic inflammatory responses, including substantially increased levels of IL-6, TNF-α, TNF receptors and monocyte chemoattractant protein-1 (MCP-1)Reference Mozaffarian, Rimm, King, Lawler, McDonald and Levy19. Furthermore, trans-fatty acids have been associated with increased levels of several markers of endothelial activation, including soluble intercellular adhesion molecule 1, soluble vascular-cell adhesion molecule 1 and E-selectinReference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu20. Trans-fatty acids are postulated to be involved in promoting vascular dysfunction, as reflected by a reduction in brachial artery flowReference de Roos, Bots and Katan21. These observations suggest that trans-fatty acids are linked to the development of CHD, probably via a vascular pro-inflammatory response.
Although there is strong epidemiological evidence implicating elevated trans-fatty acid consumption in the development of CHD, the extent and manner in which trans-fatty acids affect the vasculature remain largely unknown. Clearly, vascular endothelial cells play a vital role in the development and progression of atherogenesis. In the present study, we initiated in vitro studies to determine the direct effects of trans-fatty acid supplementation on the phenotypic and functional consequences in HAEC. We hypothesised that trans-fatty acid incorporation would induce a pro-inflammatory response leading to altered cell function.
Materials and methods
Materials
Chemicals and reagents were purchased from Sigma Chemical Company (St Louis, MO, USA), unless otherwise noted. Growth factor-reduced Matrigel matrix and antibodies coupled with fluorescent labels were purchased from Becton Dickinson (Bedford, MA, USA). Consumable tissue culture materials and Transwell inserts were acquired from Fisher Scientific (Pittsburgh, PA, USA). Sphingosine-1-phosphate (SPP) was purchased from Calbiochem (La Jolla, CA, USA). The protein growth factors utilised in the present study and the MCP-1 ELISA kits were acquired from R & D Systems, Inc. (Minneapolis, MN, USA). Human-derived aortic endothelial cells as well as the EGM-2MV Bullet kits (endothelial growth medium-2 microvascular) were purchased from Cambrex (East Rutherford, NJ, USA). All fatty acids were acquired from Nu-Chek Prep Incorporated (Elysian, MN, USA).
Human aortic endothelial cell culture
A primary cell line derived from HAEC was maintained in endothelial cell basal medium-2 (EBM-2) containing 5 % fetal bovine serum and the bullet kit materials as specified by the manufacturer. Cells were maintained at 37°C in a humidified atmosphere in the presence of 5 % CO2. Only endothelial cell cultures of less than ten passages and 80–90 % confluence were utilised in the present study.
Fatty acid incorporation into the endothelial cells
Stock solutions (1 mm) of fatty acids (cis-18 : 2, linoleic acid; trans-18 : 2, linoelaidic acid) were prepared by complexing with fatty acid-free bovine serum albuminReference van Greevenbroek, Voorhout, Erkelens, van Meer and de Bruin22. Sub-confluent endothelial cells were cultured for 24 h in EBM-2 complete media either in the presence or absence of 25 μm-cis-18 : 2 or -trans-18 : 2 fatty acid. This concentration of fatty acids was found to be optimum by time- and dose-dependent assessment of fatty acid effect on cell growth and morphology (data not shown). After incubation, the cells were trypsinised and repeatedly washed in PBS (Ca and Mg free) containing 1 % bovine serum albumin to ensure removal of NEFA. Lipids were extracted with chloroform–methanol (2:1, v/v) using the Folch methodReference Folch, Lees and Sloane-Stanley23. The lipid extracts were further fractionated into phospholipids, TAG and cholesteryl esters by TLC using a solvent system (hexane–diethyl ether–acetic acid, 70:30:1, by vol.). The lipid fractions were scraped from the TLC plate and subjected to acid-catalysed esterification by heating at 100°C for 90 min in a boron trifluoride–methanol solution (14 %). The methyl esters of fatty acids were separated on a GC system (Shimadzu GC2010; Shimadzu, Columbia, MD, USA) equipped with an Rt 2560 column (100 m; 0·25 mm internal diameter; 0·2 μm). The oven temperature was ramped from 100°C (4 min hold) to 240°C at 3°C/min (10 min hold) with a flame ionisation detector at 250°C. Fatty acid peaks were identified by retention time in comparison with authentic standards (Restek Corp., Bellefonte, PA, USA). Areas of identified peaks from 14 : 0 to 22 : 6n-3 were summed and individual fatty acids are expressed as area percentage of total identified peak areas. Data were analysed with Shimadzu's GC solutions software (Columbia, MD, USA).
Flow cytometric analysis of adhesion molecule expression
Trypsinised endothelial cells (1 × 105/sample) were washed in PBS containing 0·5 % bovine serum albumin and re-suspended into a volume of 100 μl of this labelling buffer. Cells were labelled with 0·25 μg phycoerythrin-conjugated antibody for 20 min; subsequently, the cells were washed twice in PBS containing 0·5 % bovine serum albumin. An isotype control was established for each sample set to ensure specificity of the antibody binding. Analysis was performed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with an air-cooled Ar laser emitting at a 488 nm wavelength. Fluorescence was detected through a 575 ± 26 band pass filter and quantified using CellQuest Software (Becton Dickinson). Results indicate the mean fluorescent intensity of gated endothelial cells, which excluded cellular debris and particles.
Endothelial cell adhesion to basement membrane components
HAEC (1 × 104) cultured with fatty acids, as described above, were placed onto fibronectin (5 μg/cm2) or vitronectin (1 μg/cm2) coated twenty-four-well plates. Cells were incubated for 30 min at 37°C. Aspirating cells from the wells terminated the assay; subsequently, the remaining non-adherent cells were removed by washing three times in PBS (Ca and Mg free). The adherent cells were fixed in a 5 % formaldehyde solution. Adhesion was quantified by enumerating the average number of cells observed within random fields of view (200 × ).
Leucocyte adhesion to endothelial cell monolayers
Leucocytes were isolated from normal human peripheral blood in compliance with institutional guidelines. Neutrophil leucocytes were selected by means of density gradient centrifugation using the Ficoll–Hypaque technique as previously describedReference English, Martin, Harvey, Akard, Allen, Widlanski, Garcia and Siddiqui24. Monocytes were enriched on a Ficoll–Hypaque gradient before a second density gradient centrifugation step using a 1:1 isosmotic Percoll solution with PBS–citrate (NaH2PO4, 1·49 mm; Na2HPO4, 9·15 mm; NaCl, 140 mm; C6H5Na3O7.2H2O, 13 mm; pH 7·2) as previously describedReference de Almeida, Silva, Barral and Barral25. The leucocytes were washed twice in Hanks balanced salt solution and re-suspended to a concentration of 1 × 105 cells/ml. HAEC were grown with cis-18 : 2 or trans-18 : 2 fatty acids in twenty-four-well tissue plates to near confluency before use. Cells were washed to remove fatty acids, and neutrophils or monocytes (1 × 104) were loaded onto the endothelial cell monolayers and maintained at 37°C for 30 min. Non-adherent cells were aspirated from the wells. To ensure the removal of remnant non-adherent cells, the monolayers were washed three times with Hank's balanced salt solution followed by fixation of adherent cells in a 5 % formaldehyde solution. Adhesion to the endothelial cells was quantified by enumerating the average number of leucocytes observed within random fields of view (200 × ) by at least two blinded observers. Samples were assayed in quadruplicate.
Monocyte chemoattractant protein-1 analysis by enzyme-linked immunosorbent assay
MCP-1 released from endothelial cells into the culture media was quantified using a Quantikine Human MCP-1 Immunoassay ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's guidelines.
Endothelial cell migration assay
Endothelial cell migration was performed as previously describedReference English, Kovala, Welch, Harvey, Siddiqui, Brindley and Garcia26. Briefly, harvested HAEC were washed in serum-free EBM-2 and re-suspended to a concentration of 1 × 106 cells/ml. Cells (1 × 105) were placed onto an 8 μm Transwell chamber and incubated for 30 min at 37°C to permit anchoring to the filter. These inserts were placed into wells containing 300 μl serum-free EBM-2 containing SPP to induce directed migration over a 4 h incubation. To halt the HAEC migration, cells were removed from the upper compartment and the migrated cells were fixed in a 5 % formaldehyde solution. The cells were subsequently stained with 4′,6-diamidino-2-phenylindole (5 μg/ml) to visualise the migrated cells. HAEC migration was quantified on a Leica inverted fluorescent microscope (model no. DMI4000B; Leica Microsystems, Wetzlar, Switzerland) by enumerating the average cell number in three randomly selected fields of view (200 × ) on three separate filtersReference Boguslawski, Grogg, Harvey and English27.
In vitro endothelial cell capillary morphogenesis assay
HAEC differentiation into capillary-like structures was accomplished using a two-dimensional Matrigel-based assay as previously describedReference Harvey, Welch, Kovala, Garcia and English28. Briefly, cells (3·5 × 104/well) treated with cis-18 : 2 or trans-18 : 2 fatty acids were placed into Matrigel-coated twenty-four-well tissue culture plates. The cells were incubated in the absence or presence of angiogenic stimulants (hepatocyte growth factor or SPP) and maintained for 16 h at 37°C in the presence of 5 % CO2. Non-treated control samples were maintained in serum-free EBM-2 media. Capillary-like structures were examined microscopically (40 × ) using an inverted Olympus CK40 microscope and random photomicrographs were taken. Quantification of the capillary-like structures was accomplished by enumerating the number of multi-cellular nodes as previously describedReference Harvey, Welch, Kovala, Garcia and English28, Reference Harvey, Siddiqui, Sliva, Garcia and English29. Each sample was assayed in triplicate and reproduced on at least three separate occasions.
Statistical analysis
Data are represented as mean values and standard deviations of at least three determinants. Statistical significance between datasets was determined using the Student's t test. Overall tests were performed using ANOVA. Pairwise comparisons between groups were performed using Tukey's multiple comparison test. When a calculated P value of less than 0·05 was observed, statistical significance is indicated.
Results
Fatty acid incorporation
Endothelial cells were cultured for 24 h in EBM-2 complete media either in the presence or absence of 25 μm-cis-18 : 2 or -trans-18 : 2 fatty acids. Both cis- and trans-fatty acids were incorporated in HAEC resulting in an increase in the total PUFA fraction, a corresponding decrease in the MUFA fraction and only a modest decrease in the SFA fraction (Fig. 1 (A)). Data presented in Fig. 1 (B) indicate that trans-18 : 2 fatty acids incorporated more efficiently than the cis-fatty acid counterpart. The cellular content of trans-18 : 2 increased to 21·9 (sd 1·0) % of the fatty acid content in the presence of 25 μm-trans-18 : 2; however, a similar concentration of cis-18 : 2 resulted in an increase to 13·2 (sd 1·6) % of the cellular fatty acid content. We further analysed fatty acid content in the phospholipid, TAG and cholesteryl ester fractions of endothelial cells treated with cis-18 : 2 or trans-18 : 2 fatty acids. Consistent with total cell homogenates, more trans-18 : 2 fatty acids were enriched in phospholipids than cis-18 : 2 fatty acids (cis-18 : 2 distribution: untreated, 1·9 %; cis-18 : 2-treated, 22·5 %; trans-18 : 2-treated, 1·2 %. Trans-18 : 2 distribution: untreated, 0 %; cis-18 : 2-treated, 0 %; trans-18 : 2-treated, 40·4 %). However, a similar level of cis-18 : 2 and trans-18 : 2 enrichment was observed in the TAG fraction (cis-18 : 2 distribution: untreated, 2·2 %; cis-18 : 2-treated, 16·7 %; trans-18 : 2-treated, 2·1 %. Trans-18 : 2 distribution: untreated, 0 %; cis-18 : 2-treated, 0 %; trans-18 : 2-treated, 16·7 %). No detectable amounts of cis-18 : 2 or trans-18 : 2 were found in the cholesteryl ester fractions.
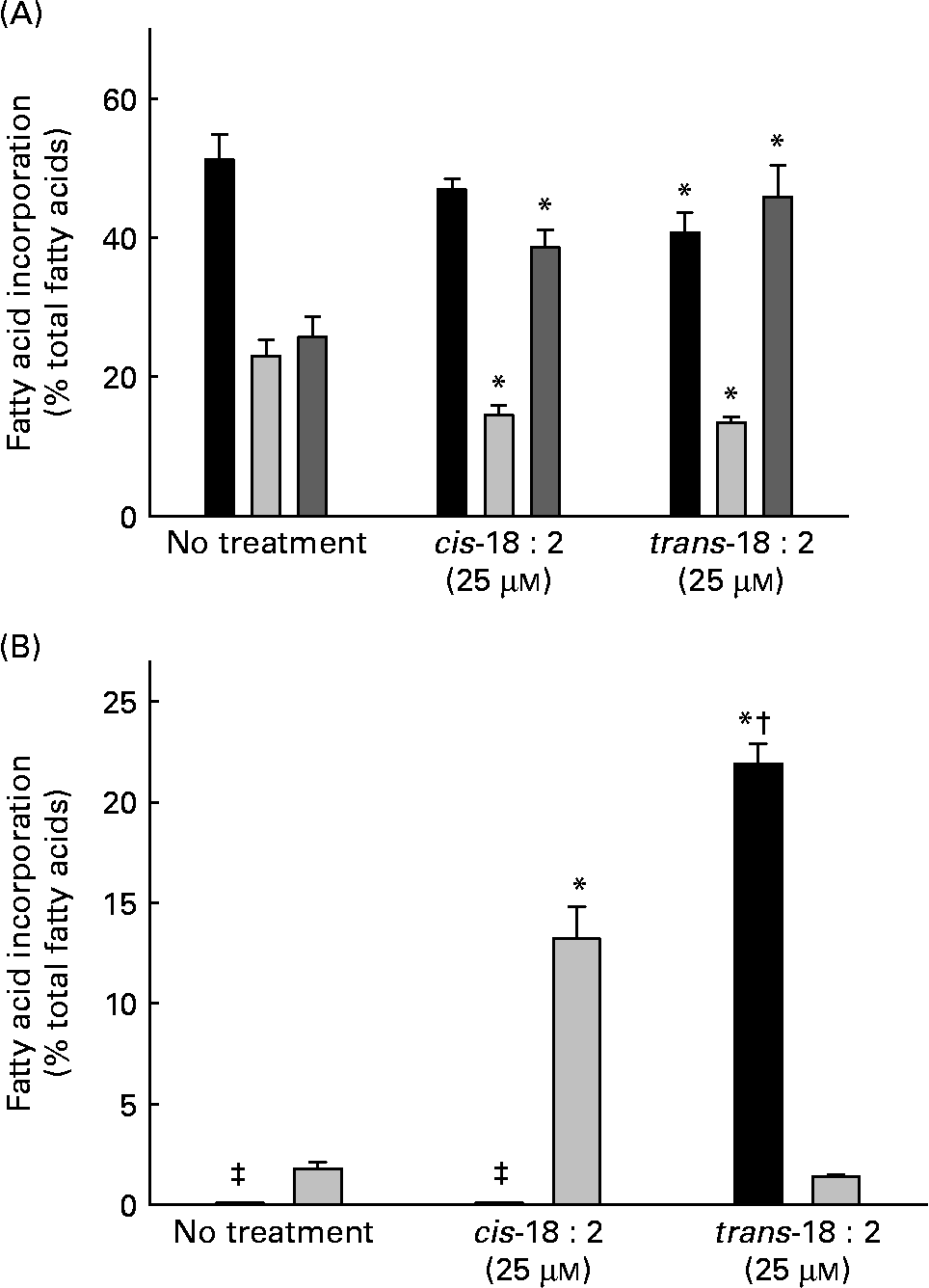
Fig. 1 Fatty acid composition of human aortic endothelial cells incorporated with cis- and trans-18 : 2 fatty acids. Sub-confluent endothelial cells were cultured for 24 h in endothelial cell basal medium-2 complete media in the presence or absence of fatty acid (25 μm). Incorporation of fatty acids was analysed by GC (Shimadzu GC2010; Shimadzu, Columbia, MD, USA). (A) Distribution of the fatty acid classes in treated endothelial cells: SFA (■), MUFA (![]() ), PUFA (
), PUFA (![]() ). (B) Relative incorporation of cis- (
). (B) Relative incorporation of cis- (![]() ) and trans-18 : 2 (■) fatty acids into endothelial cells. Results are expressed as percentage composition. Data are means for at least three experiments, with standard deviations represented by vertical bars. Data were analysed by using ANOVA (P < 0·001) and Tukey's multiple comparison test. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05). ‡ Non-detectable levels of trans-18 : 2.
) and trans-18 : 2 (■) fatty acids into endothelial cells. Results are expressed as percentage composition. Data are means for at least three experiments, with standard deviations represented by vertical bars. Data were analysed by using ANOVA (P < 0·001) and Tukey's multiple comparison test. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05). ‡ Non-detectable levels of trans-18 : 2.
Adhesion molecule expression on endothelial cells
The relative expression of adhesion molecules, intercellular adhesion molecule-1 (CD54) and vitronectin receptor (CD51/CD61) was determined in HAEC after cis-18 : 2 and trans-18 : 2 treatment using flow cytometric analysis. Intercellular adhesion molecule-1 surface expression level (mean fluorescent intensity) was 32·8 % higher in trans-18 : 2-treated cells (154·0 (sd 10·9)) compared with that of cis-18 : 2-treated cells (115·9 (sd 5·4)) (Table 1). Similarly, vitronectin receptor (CD51/CD61) expression exhibited a 21·8 % increase in the trans-18 : 2 fatty acid-treated endothelial cells (95·6 (sd 4·2)) compared with that of cis-18 : 2-treated cells (78·5 (sd 6·9)) (Table 1).
Table 1 Fatty acid effects on endothelial cell inflammatory responses†
(Mean values and standard deviations)
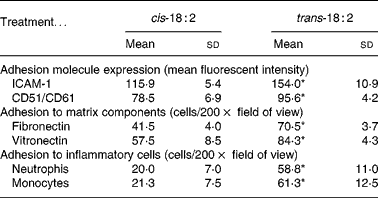
ICAM-1, intercellular adhesion molecule-1.
* Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05).
† Assays were performed as described in the text. Data were analysed using Student's t test between groups (n 4).
Human aortic endothelial cell adhesion to basement membrane components
After observing the increased expression levels of key endothelial adhesion molecules, fatty acid-incorporated HAEC were examined for their ability to bind preferentially to fibronectin- and vitronectin-coated wells. Compared with cis-18 : 2, trans-fatty acid-treated HAEC demonstrated a nearly 1·7-fold increase in their ability to adhere to fibronectin (trans-18 : 2, 70·5 (sd 3·7) v. cis-18 : 2, 41·5 (sd 4·0); Table 1). Similarly, an approximately 1·5-fold increase was observed in trans-fatty acid-treated HAEC adherence to vitronectin (Table 1).
Leucocyte adhesion to human aortic endothelial cells
Increased adhesion molecule expression on endothelial cells is often a key indicator of a pro-inflammatory state, which would result in a greater capacity for leucocyte tethering and subsequent binding and extravasation. We therefore isolated neutrophils and monocytes from normal peripheral blood to determine the effect of cis-18 : 2 and trans-18 : 2 fatty acid incorporation on leucocyte adherence to endothelial cell monolayers. Both neutrophils (trans-18 : 2, 58·8 (sd 11·0) v. cis-18 : 2, 20·0 (sd 7·0)) and monocytes (trans-18: 2, 61·3 (sd 12·5) v. cis-18 : 2, 21·3 (sd 7·5)) showed a nearly 3-fold greater adherence to trans-18 : 2-treated HAEC than that measured with cis-18 : 2-treated HAEC (Table 1).
Monocyte chemoattractant protein-1 release
In addition to the alterations of the cell membrane composition leading to increased adhesion potential, increased cytokine production, namely MCP-1, was assayed to determine the endothelial cells' ability to attract leucocytes. Supernatant fractions were harvested 24 h post-fatty acid incorporation. As shown in Fig. 2, trans-18 : 2-treated endothelial cells (6·6 (sd 0·2) ng/106 cells) released roughly two times more MCP-1 than cis-18 : 2-treated HAEC (13·9 (sd 0·6) ng/106 cells).
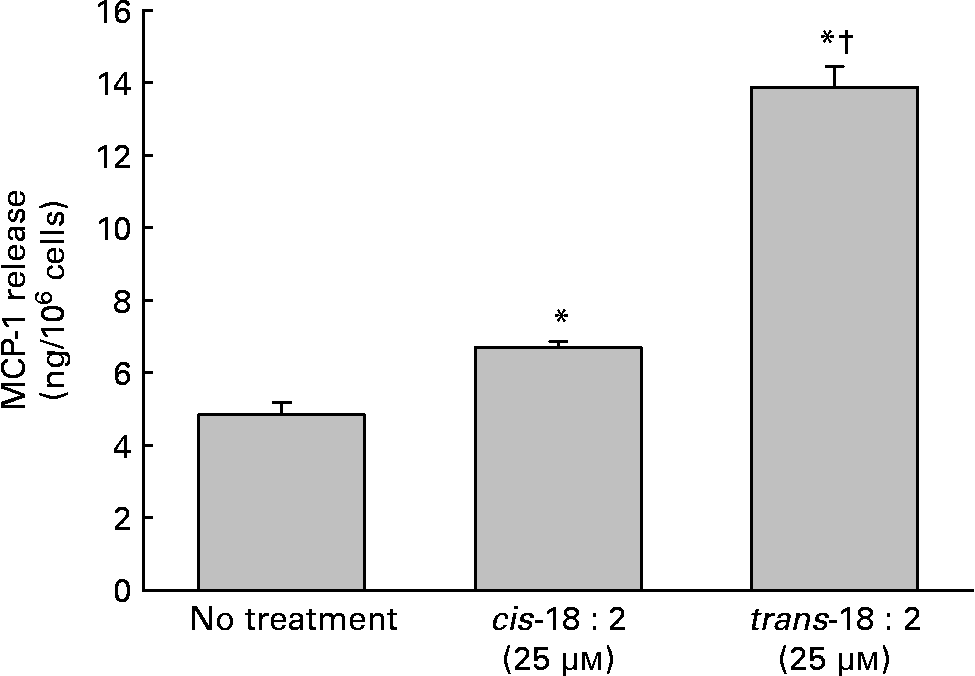
Fig. 2 Effect of cis- and trans-18 : 2 fatty acids on monocyte chemoattractant protein-1 (MCP-1) release. Endothelial cells (1 × 106) were incubated with cis- or trans-18 : 2 fatty acids for 24 h. Subsequently, the cells were washed and then incubated further with endothelial cell basal medium-2 media. Supernatant fractions were harvested 24 h post-fatty acid incorporation. MCP-1 release was quantified using a Quantikine ELISA kit purchased from R & D Systems (Minneapolis, MN, USA). Data are means for at least three experiments, with standard deviations represented by vertical bars. The data were analysed using ANOVA (P < 0·001) and Tukey's multiple comparison test. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05).
Sphingosine-1-phosphate-induced human aortic endothelial cells chemotaxis
The migratory potential of endothelial cells enriched with cis-18 : 2 or trans-18 : 2 fatty acids in response to the bioactive phospholipid SPP is presented in Fig. 3. As expected, SPP-directed migration resulted in cell mobility through the porous membrane under every condition. However, the trans-18 : 2-treated HAEC (113·3 (sd 7·0) cells/field) were 35 % more motile in response to SPP than those of cis-18 : 2-treated (83·6 (sd 5·1) cells/field) endothelial cells.
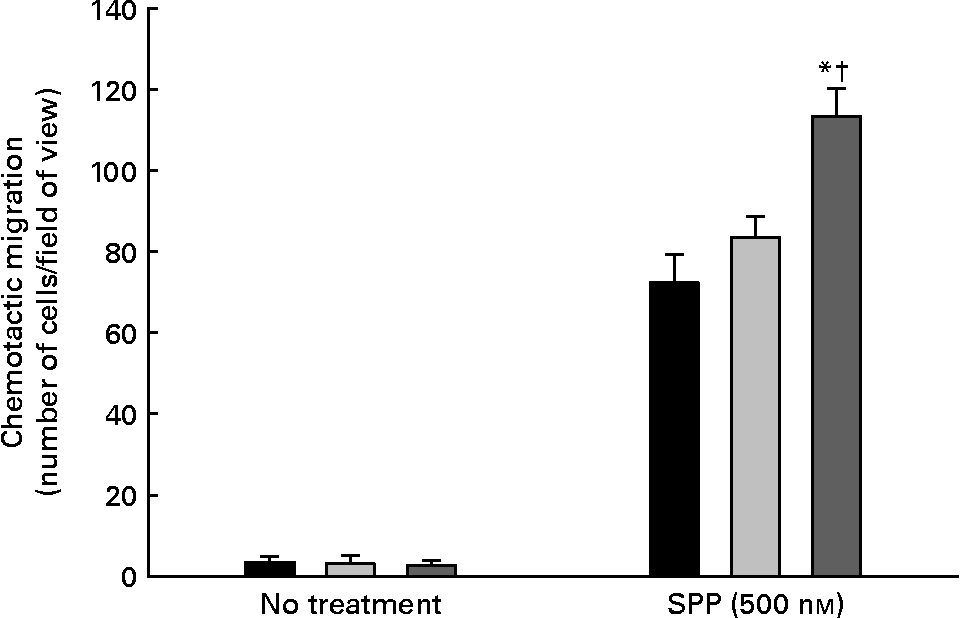
Fig. 3 Effect of cis- and trans-18 : 2 fatty acids on sphingosine-1-phosphate-induced endothelial cell chemotaxis. Endothelial cells (1 × 105) treated with cis- or trans-fatty acids for 24 h were placed onto an 8 μm Transwell chamber insert and incubated for 30 min at 37°C to permit anchoring to the filter. These inserts were then placed into wells containing serum-free endothelial cell basal medium-2 in the presence or absence of sphingosine-1-phosphate (SPP) for 4 h. The migrated cells were fixed in a 5 % formaldehyde solution and subsequently stained with 4′,6-diamidino-2-phenyindole (5 μg/ml). Human aortic endothelial cell migration was quantified on an inverted Leica fluorescent microscope by enumeration in three randomly selected fields of view (200 × ) and performed by at least two blinded individuals. (■), control; (![]() ), cis-18 : 2 (25 μm); (
), cis-18 : 2 (25 μm); (![]() ), trans-18 : 2 (25 μm). Data are means for at least three experiments, with standard deviations represented by vertical bars. The data were analysed using ANOVA (no treatment, P = 0·880; SPP-treated, P < 0·001) and Tukey's multiple comparison test in SPP-treated cells. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05).
), trans-18 : 2 (25 μm). Data are means for at least three experiments, with standard deviations represented by vertical bars. The data were analysed using ANOVA (no treatment, P = 0·880; SPP-treated, P < 0·001) and Tukey's multiple comparison test in SPP-treated cells. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05).
Human aortic endothelial cells capillary morphogenesis
Endothelial cells stimulated with pro-angiogenic phospholipids and/or protein growth factors characteristically develop into a network of capillary-like structures on Matrigel matrix supports. Fig. 4 (A) depicts a typical representation of the capillary-like structures in response to either SPP or hepatocyte growth factor. The extent of this structural formation was quantified and presented in Fig. 4 (B). Cis-18 : 2 fatty acid-incorporated endothelial cells mimicked the robust capillary morphogenic response of non-supplemented cells to both SPP and hepatocyte growth factor. HAEC supplemented with trans-18 : 2 fatty acids demonstrated an impaired ability to form the capillary-like structures on Matrigel supports and exhibited an 80 % reduction in capillary morphogenesis in the presence of SPP (trans-18 : 2, 8·3 (sd 0·6) v. cis-18 : 2, 42·7 (sd 5·5)) or hepatocyte growth factor (trans-18 : 2, 3·7 (sd 1·2) v. cis-18 : 2, 22·3 (sd 4·9)). In contrast, cis-18 : 2-treated cells' ability to form capillaries was not significantly different from that of untreated control (SPP, 45·3 (sd 2·1); hepatocyte growth factor, 23·8 (sd 3·6)).

Fig. 4 Effect of cis- and trans-18 : 2 fatty acids on endothelial cell capillary morphogenesis. Endothelial cells (1 × 105) treated with cis- or trans-fatty acids for 24 h were placed onto Matrigel-coated wells as described in the Methods section. Human aortic endothelial cells were then supplemented with either sphingosine-1-phosphate (SPP; 500 nm) or hepatocyte growth factor (HGF; 100 ng/ml) and maintained for 16 h at 37°C in the presence of 5 % CO2. (A) Random photomicrographs (40 × ) were captured to assess the extent of the formation of the capillary-like structures. (B) The capillary morphogenesis was quantified by enumerating the number of multicellular nodes. (■), Control treatment; (![]() ), cis-18 : 2 (25 μm) treatment; (
), cis-18 : 2 (25 μm) treatment; (![]() ), trans-18 : 2 (25 μm) treatment. Data are means for at least three experiments, with standard deviations represented by vertical bars. The data were analysed using ANOVA (no treatment, P = 0·017; SPP-treated, P < 0·001; HGF-treated, P < 0·001) and Tukey's multiple comparison test. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05).
), trans-18 : 2 (25 μm) treatment. Data are means for at least three experiments, with standard deviations represented by vertical bars. The data were analysed using ANOVA (no treatment, P = 0·017; SPP-treated, P < 0·001; HGF-treated, P < 0·001) and Tukey's multiple comparison test. * Mean value was significantly different from that of untreated endothelial cells (P < 0·05). † Mean value was significantly different from that of the cis-18 : 2-treated cells (P < 0·05).
Discussion
Endothelial cells are critical cellular components in the development and progression of atherosclerosis. In response to inflammatory stimuli, endothelial cells exhibit increased leucocyte adherence, which can culminate in the development of atherosclerotic plaques. In the present study, we set forth to examine the ramifications of trans-fatty acid cellular incorporation on endothelial cell phenotypic and functional characterisation. We found that the incorporation of trans-fatty acids into endothelial cells enhanced the activation state of the cells and leads to altered cell function.
During the present investigation we determined the effects of trans-18 : 2 fatty acids, which have been shown to have a positive association with CHD30, on endothelial cell function. We found that approximately 2-fold greater levels of trans-18 : 2 fatty acid were enriched in the phospholipids than that of cis-18 : 2, whereas both trans-18 : 2 and cis-18 : 2 were enriched to a similar extent in TAG fractions. This observation suggests that trans-fatty acids were incorporated to a greater extent in cellular membranes. However, the most surprising observation was that the membrane phospholipid incorporation of trans-18 : 2 was nearly 40 % of total fatty acids. This level of enrichment appears to be excessive. It has been demonstrated that fatty acids are efficiently incorporated in the phospholipids of endothelial cells. In the freshly isolated human umbilical endothelial cells, about 14 % of the total fatty acids in phospholipids are present as cis-18 : 1 fatty acid but these levels greatly increased to 22 % on culturing in the presence of fetal bovine serumReference Lagarde, Sicard, Guichardant, Felisi and Dechavanne31. Furthermore, in the human endothelial cell line EA.Hy 926, when grown in the presence of 100 μm-cis-18 : 1 fatty acids, incorporation of cis-18 : 1 in phospholipids was increased to 48 % from a baseline of 24 %Reference Kilsdonk, Dorsman, van Gent and van Tol32. Interestingly, adipose tissues of patients with peripheral artery disease contained about 27 % of total fatty acids as trans-fatty acids (21 % trans-18 : 1+6 % trans-18 : 2) and about 13 % of total fatty acids were present as trans-fatty acids (8 % trans-18 : 1+5 % trans-C18 : 2) in the atherosclerotic plaquesReference Stachowska, Dolegowska, Chlubek, Wesolowska, Ciechanowski, Gutowski, Szumiłowicz and Turowski33. The trans-fatty acids in human erythrocyte membranes range from 1 to 2 % for trans-18 : 1 and from 0·2 to 0·4 % for trans-18 : 2Reference Sun, Ma, Campos, Hankinson, Manson, Stampfer, Rexrode, Willett and Hu34, Reference Lemaitre, King, Raghunathan, Pearce, Weinmann, Knopp, Copass, Cobb and Siscovick35. Total trans-fatty acid levels (trans-18 : 1+ trans-18 : 2) in adipocytes range from 6 to 9 %Reference Bortolotto, Reis, Ferreira, Costa, Mottin, Souto and Guaragan36. Another study demonstrated that levels of total trans-fatty acids increased in the phospholipid fractions of human serum from 1 % to nearly 4 % on a trans-fatty acid-enriched diet after 4 weeksReference Vidgren, Louheranta, Agren, Schwab and Uusitupa37. The phospholipid fraction from the rat's diaphragm showed accumulation of trans-18 : 1 up to 5 % after consuming a trans-fatty acid diet for 3 months. These observations indicate that levels of trans-18 : 1 can be increased to a variable proportion in different tissues on consuming trans-fatty acid diets. There are not enough data available in the literature to compare C18 : 2 trans-fatty acid enrichment in endothelial cells in animals or human subjects on a diet rich in trans-fatty acids. It is clear from these studies using trans-18 : 1 that endothelial cells can efficiently incorporate long-chain PUFA. In the present study an excessive enrichment of trans-18 : 2 in endothelial cells appears to be unphysiological, but it remains to be seen if the extent of this enrichment can be achieved in vivo or perhaps in a human system. Furthermore, the present results indicated that endothelial cells incorporated cis- and trans-18 : 2 fatty acids at the expense of MUFA and SFA content. It is of interest to note that trans-fatty acids, although unsaturated in nature, structurally resemble SFAReference Small and Steiner38. SFA typically occupy the sn-1 position, whereas unsaturated fatty acids occupy the sn-2 position in phospholipids. The present results demonstrating that trans-fatty acids are incorporated at the expense of MUFA suggest that trans-fatty acids may be acylated on the sn-2 position of phospholipids, imparting a more saturated and hydrophobic character. Although the determination of sn-1 v. sn-2 incorporation was beyond the scope of the present investigation, more saturated phospholipids, especially those containing trans-fatty acids, are known to attract cholesterolReference Niu, Mitchell and Litman39. This phenomenon plausibly alters cell membrane structure, including redefining lipid raft and non-raft regions in size, organisation and composition. Lipid rafts are important for cellular signalling, as they provide docking sites for receptors, co-receptors and mediators including adhesion moleculesReference Brown40. Our data also support this interpretation by demonstrating that cell surface expression of adhesion molecules was greatly enhanced in cells grown in the presence of trans-18 : 2 fatty acids.
HAEC adhesion molecule expression consistently corresponded with endothelial cell adherence to basement membrane components and leucocyte binding to the endothelium. Enhanced adhesion molecule expression is often associated with an inflammatory endothelial cell phenotype. Although the elevated antigenic expression levels in trans-fatty acid-treated HAEC were statistically significant, the increase was modest in comparison with an acute cytokine-stimulated response. However, the present study suggests that long-term exposure of trans-fatty acids to the endothelium could result in a gradual, cumulative chronic state of activation, which could promote the development of atherosclerosis. Additional evidence was found in the notable increase in the MCP-1 released by trans-fatty acid-treated HAEC. This modest increase in cytokine production, which attracts leucocytes to the primed endothelium, could initiate a cellular infiltration of macrophages, thereby initiating a cascade of plaque formation and intimal thickening. Increased MCP-1 cytokine production has been correlated with the prevalence of atherosclerosisReference Takeya, Yoshimura, Leonard and Takahashi41.
Previous studies have demonstrated that SPP exerts pro-angiogenic effects on endothelial cells, including increases in barrier integrity, chemotaxis and capillary morphogenesisReference English, Kovala, Welch, Harvey, Siddiqui, Brindley and Garcia26, Reference Harvey, Siddiqui, Sliva, Garcia and English29, Reference English, Welch, Kovala, Harvey, Volpert, Brindley and Garcia42, Reference Garcia, Liu, Verin, Birukova, Dechert, Gerthoffer, Bamberg and English43. SPP-induced chemotaxis in endothelial cells was further enhanced in the trans-fatty acid-treated HAEC. Following migration to the site of wound healing, endothelial cells differentiate into vessel linings, a process mimicked in vitro by the assessment of capillary morphogenesis on Matrigel matrix supportsReference Harvey, Siddiqui, Sliva, Garcia and English29. The SPP-induced capillary-like structural formation was significantly impaired in the trans-fatty acid-treated endothelial cells. This endothelial dysfunction could translate into an inability of endothelial cells to repair damaged vessel linings, complicating the pathogenesis of the arterial damage. Furthermore, this process could explain impairment in collateral growth that serves to compensate for an arterial occlusion, especially in the coronary circulation. Thus, trans-fatty acids may play an important role in the development of CHD, and perhaps peripheral vascular disease, through by inhibiting compensatory remodelling.
Endothelial cell apoptosis has been implicated in the progression of atherosclerosis, possibly even contributing to the rupturing of atherosclerotic plaquesReference Mallat and Tedgui44. A recent report by Zapolska-Downar et al. Reference Zapolska-Downar, Kosmider and Naruszewicz45 demonstrated that trans-fatty acids induce endothelial cell apoptosis, which is consistent with an effect of trans-fatty acids on the latter stages of plaque development and/or subsequent rupturing of the plaques. The induction of endothelial cell apoptosis observed by Zapolska-Downar et al. required significantly higher trans-fatty acid supplementation (up to 5 mm). Using considerably lower trans-fatty acid-treatments (25 μm) under the same 24 h time frame, we were unable to observe an increase in early signs of apoptosis in HAEC using Annexin V–propidium iodide staining techniques (data not shown). The trans-fatty acid-induced alterations in endothelial cell activation and function in the present study are clearly not due to the initiation of apoptosis. These alterations implicate trans-fatty acids in triggering the development of atherosclerosis and/or accelerating the progression of the disease. Trans-fatty acids may impart their effect by enhancing intrinsic signalling mechanisms leading to a chronic, pro-inflammatory state.
In an investigation by Kummerow et al. Reference Kummerow, Zhou and Mahfouz46 trans-fatty acid incorporation into HAEC resulted in increased Ca influx in combination with Mg depletion. Both linoelaidic (trans-18 : 2) and elaidic (trans-C18 : 1) acids increased incorporation of radiolabelled Ca intracellularly, whereas stearic (C18 : 0) and oleic (cis-18 : 2) acids did not. The authors suggest that this model is representative of endothelial cell calcification, a hallmark characteristic of atherosclerosis, and that dietary trans-fatty acids compound the effect of the relatively low-Mg American diet on this process. While these modest increases in Ca influx probably result from an alteration in cell membrane fatty acid composition and properties, the effect of free trans-fatty acids on endothelial cell function were not included in their study.
Consumption of trans-fatty acids was correlated with adverse affects on endothelial cell function in vivo Reference Mozaffarian, Rimm, King, Lawler, McDonald and Levy19, Reference Mozaffarian47. Increased plasma concentrations of biomarkers of inflammation, including soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1 and E-selectin, were associated with the trans-fatty acid content of the diet in the Nurses' Health Study, a cross-sectional investigation of 730 CVD-free womenReference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu20. The authors suggest that this association could explain the significantly greater risk of developing CVD based on the consumption of a high-trans-fatty acid content diet. While soluble adhesion molecules and inflammatory cytokines correlate with CVD in vivo Reference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu20, Reference Kaul48, multiple factors could trigger such a response as a result of the progression of the disease.
In conclusion, the present study provides evidence for a direct effect of trans-18 : 2 incorporation on the activation status and functional consequence of endothelial cells in vitro, in the absence of stimulation factors found in plasma. Consumption of diets high in trans-fatty acids may induce long-term progressive changes in the endothelium that could trigger the development of CVD. The present study suggests minimising or eliminating the dietary intake of trans-fatty acids might prevent the initiation of a pro-inflammatory state leading to the subsequent development of atherosclerosis. We realised that cis-18 : 2 and trans-18 : 2 fatty acids were incorporated to a different extent in endothelial cells when incubated with a similar concentration (25 μm) of these fatty acids, which was an unexpected finding. It is possible that the altered biological activities in trans-18 : 2-treated endothelial cells we observed were simply due to a higher content of fatty acids and independent of their geometric isomers. Further investigation is required to study biological activities related to inflammation in endothelial cells after incorporating similar levels of fatty acids.
Acknowledgements
The authors wish to thank Mr Colin Terry for the statistical analysis of the data and Dr Karen Spear for her editorial assistance. The contract grant sponsor was Showalter Cardiovascular Fund (contract grant number S-2007-1).







