PPAR represent a subfamily of nuclear hormone receptors that are activated by a variety of dietary and endogenous fatty acids (Schoonjans et al. Reference Schoonjans, Staels and Auwerx1996; Xu et al. Reference Xu, Lambert and Montana1999). The PPAR family is currently divided into three subgroups: α, β/δ, and γ (Daynes & Jones, Reference Daynes and Jones2002). These subgroups are characterised by distinct patterns of tissue distribution and metabolic function (Braissant et al. Reference Braissant, Foufelle, Scotto, Dauca and Wahli1996; Schoonjans et al. Reference Schoonjans, Martin, Staels and Auwerx1997). Some PPARγ agonists, such as 15-deoxy-Δ12,14-prostaglandin J2 and the insulin-sensitising thiazolidinediones, have been reported to exert anti-inflammatory actions by reducing inflammation-associated molecules (Ricote et al. Reference Ricote, Li, Willsons, Kelly and Glass1998). The PPARγ agonists, 15-deoxy-Δ12,14-prostaglandin J2 and troglitazone, inhibit phorbol myristyl acetate-induced production of IL-1β, IL-6, and TNF-α in peripheral blood monocytes (Jiang et al. Reference Jiang, Ting and Seed1998). However, pioglitazone and rosiglitazone, which are also PPARγ agonists, do not inhibit phorbol ester-induced TNF-α release from human monocytic THP-1 cells (Cunard et al. Reference Cunard, Ricote, DiCampli, Archer, Kahn, Glass and Kelly2002). Although PPARγ was originally shown to play a key role in adipocyte differentiation and lipid metabolism (Chawla et al. Reference Chawla, Schwarz, Dimaculangan and Lazar1994), it potentially may play an important role in regulating inflammatory and immune responses by modulating the activity of monocytes and macrophages.
In clinical medicine, nutritional therapy may be important for immunoregulation, inflammation, neoplasia and vascular diseases (Calder, Reference Calder1998). Modulation of the immune system by dietary fatty acids may occur through regulation of arachidonic acid metabolism and eicosanoid production (Calder, Reference Calder1996; Miles et al. Reference Miles, Allen and Calder2002), modification of membrane fluidity (Mexmain et al. Reference Mexmain, Gualde, Aldigier, Motta, Chable-Rabinovitch and Rigaud1984) and transcriptional regulation of gene expression by PPAR (Bishop-Bailey & Wray, Reference Bishop-Bailey and Wray2003). Conjugated linoleic acid (CLA), a dietary fatty acid, has received special attention since it can trigger modification of immune cell functions, as well as having numerous other physiological characteristics such as anti-adipogenic (Smedman & Vessby, Reference Smedman and Vessby2001), anti-diabetogenic (Ryder et al. Reference Ryder, Portocarrero and Song2001), anti-carcinogenic (Palombo et al. Reference Palombo, Gangguly, Bistran and Menard2002) and anti-atherosclerotic (Lee et al. Reference Lee, Kritchevsky and Pariza1994) properties. Recently, the putative detrimental influences of CLA have been reported. CLA acts as a cancer promoter in colon carcinogenesis (Rajakangas et al. Reference Rajakangas, Basu, Salminen and Mutanen2003) and increases inflammatory indicators such as C-reactive protein (Smedman et al. Reference Smedman, Basu, Jovinge, Fredrikson and Vessby2005). It causes significant impairment of peripheral insulin sensitivity as well as of blood glucose and serum lipid concentrations (Risérus et al. Reference Risérus, Smedman, Basu and Vessby2004).
CLA can stimulate or inhibit immune cell function. CLA increases TNF-α and IL-6 secretion and decreases IL-4 secretion by splenocytes (Kelley et al. Reference Kelley, Warren, Simon, Bartolini, Mackey and Erickson2002). Expression of PPARγ is enhanced in CLA-fed pigs (Meadus et al. Reference Meadus, Maclnnis and Dugan2002). CLA is capable of modulating pro-inflammatory signals such as TNF-α and inducible NO synthase in macrophages. The regulation of inducible NO synthase transcription by CLA requires PPARγ, as demonstrated using a dominant negative construct (Yu et al. Reference Yu, Correll and Vanden Heuvel2002). CLA treatment ameliorates the symptoms of type α diabetes mellitus in Zucker diabetic fa/fa rats and affects differentiation of adipose tissue (Houseknecht et al. Reference Houseknecht, Vanden Heuvel, Moya-Camarena, Portocarrero, Peck, Nickel and Belury1998). In contrast, CLA down regulates the expression of PPARγ and its target genes, fatty-acid binding protein, and liver X receptor α in adipocytes (Granlund et al. Reference Granlund, Juvet, Pederson and Nebb2003). These reports indicate that the effects of CLA in modulating immune responses are similar to effects induced by ligands of PPARγ.
In particular, trans-10, cis-12 (t10c12)-CLA, a CLA isomer, is known to alter immune function (Pariza et al. Reference Pariza, Park and Cook2001). In previous studies, it has been shown that direct treatment with t10c12-CLA has no effect on the phagocytic capacity of porcine peripheral blood polymorphonuclear cells (PMN). However, the phagocytic capacity of porcine PMN is markedly enhanced by the culture supernatant fraction from porcine peripheral blood mononuclear cells (PBMC) treated with t10c12-CLA (Kang et al. Reference Kang, Kim, Chung, Lee and Yang2004). Therefore, it has been hypothesised that t10c12-CLA involves the production of soluble factor(s) including TNF-α, a phagocytosis-promoting factor, from porcine PBMC that enhance the phagocytic capacity of porcine PMN, which may be an important mechanism for the enhancement of the innate immune response.
In the present study, we examined whether t10c12-CLA stimulates PPARγ expression in porcine PBMC and, if so, whether activation of PPARγ in PBMC by t10c12-CLA affects TNF-α production, which enhances the phagocytic capacity of PMN. In addition, the effects of PPARγ antagonism on TNF-α production by PBMC and the phagocytic capacity of PMN were examined.
Materials and methods
Pigs
Healthy 5-month-old Landrace crossbred pigs were used as blood donors. Pigs were kept in a temperature-controlled room with a 12 h light–dark cycle and fed a commercial diet (Fildmaster; Purina Korea, Seoul, Korea) and tap water. All experimental procedures and animal use were approved by the ethics committee of the Chungbuk National University (South Korea).
Reagents and antibodies
t10c12-CLA (98 % purity) was purchased commercially (Matreya Inc., Pleasant Gap, PA, USA). t10c12-CLA stock solution was prepared by dissolving t10c12-CLA in dimethyl sulfoxide (Moya-Camarena et al. Reference Moya-Camarena, Vanden Heuvel, Blanchard, Leesnitzer and Belury1999; Brown et al. Reference Brown, Boysen, Jensen, Morrison, Storkson, Lea-Currie, Pariza, Mandrup and McIntosh2003) to a final concentration of 50 mm and passed through a 0·45 μm membrane filter (Millipore Co., Bedford, MA, USA) before use. Recombinant porcine (rp) TNF-α, goat anti-rpTNF-α polyclonal antibody (pAb) (R&D Systems Inc., Minneapolis, MN, USA), goat anti-recombinant human (rh) IL-2 pAb (Sigma-Aldrich Co., St Louis, MO, USA), and bisphenol A diglycidyl ether (BADGE) (Fluka Chemie AG, Buchs, Switzerland) were purchased commercially.
Isolation of peripheral blood mononuclear cells and polymorphonuclear cells
Heparinised porcine peripheral blood was drawn from the anterior vena cava and diluted with an equal volume of PBS without Ca and Mg and overlayed 1:1 on a Histopaque solution (specific gravity, 1·080; Sigma-Aldrich Co.). After centrifugation at 400 g for 45 min at room temperature, the cells in the interface between the plasma in PBS and the Histopaque solution were harvested and treated with 0·83 % NH4Cl in a tri(hydroxymethyl)-aminomethane-base buffer (pH 7·2) for 5 min. The resulting PBMC were washed three times with PBS. PMN were obtained from the upper layer of sedimented erythrocytes after the removal of the PBMC layer. The erythrocytes were allowed to sediment for 60 min with dextran (molecular weight, 200 000; Wako Ltd, Osaka, Japan). The floating cells were gently collected and pelleted by centrifugation at 400 g for 5 min. The residual erythrocytes were lysed by transitory treatment with 0·83 % NH4Cl. The purity of neutrophils in the final PMN suspension was routinely greater than 95 % as determined by cytospin smear and Wright–Giemsa stain. Both PBMC and PMN were re-suspended in RPMI 1640 medium (Sigma-Aldrich Co.) supplemented with 2 mm-l-glutamine, gentamicin (0·02 mg/ml), and 5 % heat-inactivated fetal bovine serum (Invitrogen Co., Grand Island, NY, USA).
Treatment of peripheral blood mononuclear cells
t10c12-CLA and/or BADGE (100 μm), a PPARγ antagonist, were added to freshly isolated PBMC culture media with a minimal volume ( < 0·1 %) of dimethyl sulfoxide as the solvent and the same amount as vehicle dimethyl sulfoxide was added to control cells. To determine mRNA expression in cells, PBMC at a density of 2 × 106 cells/ml per well in a twenty-four-well plate (Nunc Co., Naperville, IL, USA) were incubated for 3 h at 37°C under a 5 % CO2-humidified atmosphere. After incubation, the samples were aspirated and centrifuged at 400 g for 30 min at 4°C. The pelleted PBMC were stored at − 70°C until use in further analysis. The PBMC were also incubated for 24 h to obtain the PBMC culture supernatant fraction. The supernatant fractions were centrifuged at 5000 g for 30 min at 4°C, filtered with a 0·45 μm pore membrane filter, and stored at − 70°C. At the end of the incubation period, the viability of cells was consistently more than 95 % as determined by trypan blue dye exclusion.
Phagocytosis assay
PMN (100 μl), adjusted to 1 × 107 cells/ml, were added to each well of a twenty-four-well plate. The PMN were incubated with the culture supernatant fraction from PBMC that had been treated with either various concentrations of t10c12-CLA or t10c12-CLA in combination with BADGE (100 μm) for 12 h at 37°C under a 5 % CO2-humidified atmosphere. Fluorescein isothiocyanate (FITC)-latex beads (20 μl; 1 × 109 beads/ml; bead size 2·0 μm; Polysciences Inc., Warrington, PA, USA) were added to each well for the final 1 h. PMN incubated without FITC-latex beads were used as a negative control. The cultured cells were gently harvested, centrifuged at 400 g for 3 min at 4°C and washed three times with PBS containing 3 mm-EDTA. After washing three times, the supernatant fraction was discarded and replaced with PBS containing 1 % paraformaldehyde to stabilise the cells. Surface-adherent FITC fluorescence on PMN was quenched by the addition of 20 μl of 0·4 % trypan blue solution to each tube before flow cytometry analysis as previously described (Hed et al. Reference Hed, Hallden, Johansson and Larsson1987). The use of trypan blue solution has an effect in fluorescence quenching on attached particles but not ingested particles (Antal-Szalmás et al. Reference Antal-Szalmás, Poppelier, Broekhuizen, Verhoef, van Strijp and van Kessel2000). Phagocytosis was measured by FACS Calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) flow cytometry using CELLQuest software. The Ar laser was set to emit an excitation wavelength of 488 nm. FITC (green) fluorescence was measured between 515 and 560 nm on 10 000 PMN per sample. PMN were gated by forward and side light scatter characteristics. The PMN were monitored by propidium iodide (Sigma-Aldrich Co.) for live cells gated by flow cytometry. The results are expressed as percentages of absolute phagocytic capacity.
Neutralisation test
For the neutralisation test, anti-rpTNF pAb was diluted to various concentrations and added to the t10c12-CLA-stimulated PBMC culture supernatant fraction. Goat anti-rhIL-2 pAb was used as a control isotype IgG. The mixed samples were kept for 30 min at room temperature.
Measurement of tumour necrosis factor-α in the peripheral blood mononuclear cells culture supernatant fraction
Porcine PBMC were incubated with t10c12-CLA alone or t10c12-CLA plus BADGE (100 μm). The culture supernatant fraction was collected and the amount of TNF-α was determined by a direct sandwich ELISA using the Quantikine® P porcine TNF-α immunoassay kit (R&D Systems Inc.) according to the manufacturer's protocol. All samples, standards and controls were assayed in triplicate. The optical density was determined using an automated microplate reader (EL × 808; BioTek Instruments Inc., Winooski, VT, USA) at 450 nm. TNF-α was quantified from eight titration points using standard curves generated with purified porcine TNF-α, and the concentrations were expressed as pg/ml. Lower and upper detection limits were 11·7 and 1500 pg/ml, respectively.
Ribonucleic acid preparation and reverse transcriptase-polymerase chain reaction
Total RNA was prepared from porcine PBMC according to a protocol for single-step RNA isolation based on acid guanidinium-thiocyanate-phenol-chloroform extraction, using TRIzol reagent (Invitrogen Co., Carlsbad, CA, USA) as previously described (Yang et al. Reference Yang, Lee, Yun, Kim, Ko and Jeung2002). Total RNA (2 μg) was reverse transcribed into cDNA using the Moloney-murine leukaemia virus RT (Ambion Inc., Austin, TX, USA) and random primers (9-mers). To determine the conditions under which PCR amplification of PPARγ, TNF-α and cytochrome c oxidase subunit (1A) mRNA were in the logarithmic phase, samples (1 μl) were amplified using different numbers of cycles. The 1A gene was PCR-amplified to rule out the possibility of RNA degradation and to control for variations in mRNA concentrations in the RT reaction. PCR products and amplification cycles were linearly related for PPARγ, TNF-α and 1A mRNA. Thirty cycles for PPARγ and TNF-α and twenty-five cycles for 1A were employed for quantification. The cDNA were amplified in 20 μl PCR reactions containing 1 unit Taq polymerase (Promega Co., Madison, WI, USA) and its buffer, 1·5 mm-MgCl2, 2 mm dNTP and 50 pmol of specific primers. PCR reactions were denatured at 95°C for 1 min, annealed at 50°C for 1 min, and extended at 72°C for 1 min, 30 s. cDNA sequences of mRNA were obtained by RT reaction primers (Macrogen Inc., Seoul, Korea). The oligonucleotides for PPARγ were based on the cDNA sequence (GenBank accession number AJ006756): 5′-CTG GCA AAG CAC TTG TAT G-3′ (sense) and 5′-GGT GTA AAT GAT CTC GTG GA-3′ (anti-sense). The oligonucleotides for TNF-α were based on the cDNA sequence (GenBank accession number X57321): 5′-CAA GGA CTC AGA TCA TCG TC-3′ (sense) and 5′-CTT GGT CTG GTA GGA GAC G-3′ (anti-sense). The primers for the 1A gene (GenBank accession number AF03253) were 5′-CAC CGT AGG AGG TCT AAC G-3′ (sense) and 5′-GTA TCG TCG AGG TAT TCC G-3′ (anti-sense). Of the PCR products, 10 μl were fractionated on a 2 % agarose gel and stained with ethidium bromide. The photograph was scanned and analysed using the molecular analysis program Gel Doc 1000 version 1.5 (Bio-Rad Lab., Hercules, CA, USA).
Statistical analysis
All statistical analyses were carried out using the software SigmaStat version 2.03 (SPSS Inc., Chicago, IL, USA) or SAS version 9.1 (SAS Institute Co., Cary, NC, USA). One-way ANOVA was used to investigate differences between control and concentrations of CLA treatment, followed by Dunnett's post hoc test. Comparisons of two groups at each CLA concentration were made by Student's t test, separately. P < 0·05 was considered statistically significant. Data were expressed as mean values and standard deviations.
Results
Effect of trans-10, cis-12-conjugated linoleic acid on tumour necrosis factor-α expression and production in porcine peripheral blood mononuclear cells
TNF-α mRNA expression in PBMC in response to t10c12-CLA was examined. The isolated PBMC were treated with t10c12-CLA (2·5–20 μm) and harvested for total RNA extraction 3 h later. TNF-α mRNA expression in PBMC was slightly increased by t10c12-CLA and peaked at a concentration of 5 μm (Fig. 1 (A)). t10c12-CLA induced a significant increase (P < 0·001) in TNF-α production by PBMC in a dose-dependent manner (Fig. 1 (B)).

Fig. 1 TNF-α expression and production in porcine peripheral blood mononuclear cells (PBMC) by trans-10, cis-12-conjugated linoleic acid (t10c12-CLA). (A) RT-PCR analysis of TNF-α mRNA expression in porcine PBMC treated with t10c12-CLA at the indicated concentrations for 3 h (a). Normalisation of TNF-α mRNA expression with 1A (b). Signals were quantified with a molecular analysis program and expressed as a percentage of the maximum values (c). The expected product sizes of TNF-α and 1A mRNA are 273 and 277 bp, respectively. (B) The amount of TNF-α in the porcine PBMC culture supernatant fraction treated with t10c12-CLA at the indicated concentrations for 24 h was determined using an ELISA (see p. 118). One-way ANOVA was used to investigate differences between control and concentrations of CLA treatment, followed by Dunnett's post hoc test. Data are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that for the vehicle treatment (0 μm-t10c12-CLA) (P < 0·05).
Tumour necrosis factor-α increases the phagocytic capacity of porcine polymorphonuclear cells
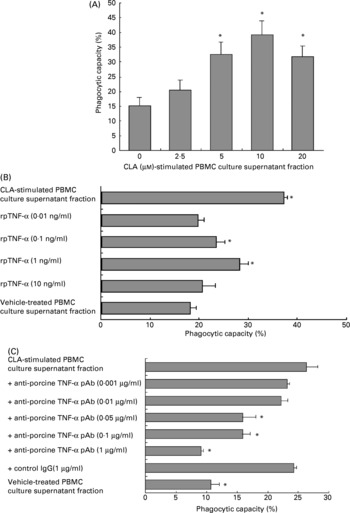
To examine the phagocytic capacity of PMN exposed to the culture supernatant fraction from PBMC treated with t10c12-CLA, PMN were incubated for 12 h with the culture supernatant fraction from PBMC treated with 2·5–20 μm-t10c12-CLA. The phagocytic capacity of PMN was significantly enhanced (P < 0·001) in a dose-dependent manner by the culture supernatant fraction (Fig. 2 (A)). Its capacity was also significantly increased (P < 0·001) in a dose-dependent manner by the addition of rpTNF-α (Fig. 2 (B)). Anti-rpTNF-α pAb neutralised the enhancement of PMN phagocytic capacity by the PBMC culture supernatant fraction treated with t10c12-CLA (10 μm) (P < 0·001) in a dose-dependent manner (Fig. 2 (C)). There was no non-specific inhibition by an immunoglobulin IgG isotype of anti-rpTNF-α pAb, since the enhanced phagocytic capacity was not inhibited by the addition of a high concentration (1 μg/ml) of the anti-rhIL-2 pAb used as a control IgG.

Fig. 2 The phagocytic capacity of porcine polymorphonuclear cells (PMN). (A) The effect of the culture supernatant fraction from porcine peripheral blood mononuclear cells (PBMC) treated with trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) on phagocytic capacity of PMN. Freshly isolated PMN (1 × 106 cells/ml per well) were incubated for 12 h with the culture supernatant fraction from PBMC (2 × 106 cells/ml) that had been treated with t10c12-CLA at the indicated concentrations for 24 h. One-way ANOVA was used to investigate differences between control and treatments, followed by Dunnett's post hoc test. Data are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that for the vehicle (0 μm-t10c12-CLA)-treated PBMC culture supernatant fraction (P < 0·05). (B) The effect of recombinant porcine (rp) TNF-α on the phagocytic capacity of PMN. PMN (1 × 106 cells/ml per well) were treated with rpTNF-α at the indicated concentrations for 12 h. The culture supernatant fraction from PBMC (2 × 106 cells/ml) treated with t10c12-CLA (10 μm) for 24 h was prepared as a positive control. One-way ANOVA was used to investigate differences between control and treatments, followed by Dunnett's post hoc test. Data are means (n 6), with standard deviations represented by horizontal bars. * Mean value was significantly different from that for the vehicle-treated PBMC culture supernatant fraction (P < 0·05). (C) The neutralising effect of anti-rpTNF-α polyclonal antibody (pAb) on the phagocytic capacity of PMN. Anti-rpTNF-α pAb, at the indicated concentrations, was added to the culture supernatant fraction from PBMC (2 × 106 cells/ml) treated with t10c12-CLA (10 μm) for 24 h. Goat anti-recombinant human IL-2 pAb (1 μg/ml) was used as a control isotype IgG. The mixed samples were kept for 30 min. Fluorescein isothiocyanate-latex beads were added to all cultures for the final 1 h. The phagocytic capacity of PMN was measured using flow cytometry (see pp. 118-119). One-way ANOVA was used to investigate differences between control and treatments, followed by Dunnett's post hoc test. Data are means (n 6), with standard deviations represented by horizontal bars. * Mean value was significantly different from that for the t10c12-CLA-treated PBMC culture supernatant fraction (P < 0·05).
Effect of trans-10, cis-12-conjugated linoleic acid on peroxisome proliferator-activated receptor-γ mRNA expression in porcine peripheral blood mononuclear cells
To examine the expression of PPARγ mRNA in PBMC in response to t10c12-CLA, PBMC were incubated with t10c12-CLA and harvested for RNA isolation. Porcine PPARγ mRNA expression in PBMC was increased in a dose-dependent manner by t10c12-CLA treatment and peaked at 5 μm-t10c12-CLA, although a detectable PPARγ signal was seen in the vehicle-treated PBMC after 3 h of incubation (Fig. 3).

Fig. 3 PPARγ mRNA expression in porcine peripheral blood mononuclear cells (PBMC) after trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) treatment. RT-PCR analysis of PPARγ mRNA expression in PBMC treated with t10c12-CLA at the indicated concentrations for 3 h (a). Normalisation of the PPARγ mRNA expression with 1A (b). Signals were quantified with a molecular analysis program and expressed as a percentage of the maximum values (c). The expected product sizes of PPARγ and 1A mRNA are 345 and 277 bp, respectively.
Effects of the peroxisome proliferator-activated receptor-γ antagonist bisphenol A diglycidyl ether on tumour necrosis factor-α expression in peripheral blood mononuclear cells and the phagocytic capacity of polymorphonuclear cells
To examine whether the activation of PPARγ by t10c12-CLA was associated with TNF-α production by PBMC and the phagocytic capacity of PMN, BADGE, a PPARγ antagonist, was added to the PBMC culture. The amount of PBMC TNF-α production and the stimulation of PMN phagocytic capacity by the culture supernatant fraction from PBMC treated with t10c12-CLA alone or in combination with BADGE (100 μm) for 24 h were determined. As shown in Fig. 4 (A), BADGE completely negated (P < 0·001) the effect of t10c12-CLA on TNF-α production by PBMC when compared with the effect of the culture supernatant fraction without BADGE. The enhanced phagocytic capacity of PMN in response to the culture supernatant fraction from PBMC treated with t10c12-CLA (5–20 μm) was inhibited (P < 0·001) by the addition of BADGE to the PBMC culture (Fig. 4 (B)). RT-PCR analysis also showed that BADGE completely antagonised the TNF-α mRNA expression induced by t10c12-CLA in PBMC (Fig. 4 (C)).

Fig. 4 Effects of bisphenol A diglycidyl ether (BADGE), a PPARγ antagonist, on TNF-α expression in peripheral blood mononuclear cells (PBMC) and the phagocytic capacity of polymorphonuclear cells (PMN). (A) The amount of TNF-α in the culture supernatant fraction from PBMC (2 × 106 cells/ml) treated with either trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) alone (![]() ) or t10c12-CLA in combination with BADGE (□) at the indicated concentrations for 24 h was determined using ELISA. (B) The phagocytic capacity of PMN in response to the culture supernatant fractions from PBMC (2 × 106 cells/ml) treated with either t10c12-CLA alone or t10c12-CLA in combination with BADGE, at the indicated concentrations for 24 h. PMN (1 × 106 cells/ml per well) were incubated for 12 h. Cultures were supplemented with fluorescein isothiocyanate-latex beads for the final 1 h. Differences within the input groups of each t10c12-CLA concentration were analysed with Student's t test, separately. Data are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that for the culture supernatant fraction from PBMC treated with t10c12-CLA alone (P < 0·05). (C) The effect of BADGE on TNF-α mRNA expression in porcine PBMC treated with t10c12-CLA for 3 h. RT-PCR analysis of TNF-α mRNA expression in PBMC treated with t10c12-CLA (a). Normalisation of TNF-α mRNA expression with 1A (b). The expected product sizes of TNF-α and 1A mRNA are 273 and 277 bp, respectively.
) or t10c12-CLA in combination with BADGE (□) at the indicated concentrations for 24 h was determined using ELISA. (B) The phagocytic capacity of PMN in response to the culture supernatant fractions from PBMC (2 × 106 cells/ml) treated with either t10c12-CLA alone or t10c12-CLA in combination with BADGE, at the indicated concentrations for 24 h. PMN (1 × 106 cells/ml per well) were incubated for 12 h. Cultures were supplemented with fluorescein isothiocyanate-latex beads for the final 1 h. Differences within the input groups of each t10c12-CLA concentration were analysed with Student's t test, separately. Data are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that for the culture supernatant fraction from PBMC treated with t10c12-CLA alone (P < 0·05). (C) The effect of BADGE on TNF-α mRNA expression in porcine PBMC treated with t10c12-CLA for 3 h. RT-PCR analysis of TNF-α mRNA expression in PBMC treated with t10c12-CLA (a). Normalisation of TNF-α mRNA expression with 1A (b). The expected product sizes of TNF-α and 1A mRNA are 273 and 277 bp, respectively.
Discussion
t10c12-CLA up regulated TNF-α production from porcine PBMC. The phagocytic capacity of porcine peripheral blood PMN was increased by the culture supernatant fraction from PBMC treated with t10c12-CLA. The phagocytic capacity of PMN was also increased by rpTNF-α dose-dependently. It was highest at the rpTNF-α concentration of 1 ng/ml and down regulated by 10 ng/ml. Similar to the phagocytic capacity kinetics of rpTNF-α, the rhTNF-α concentration of 1 ng/ml exhibited the highest capacity in porcine PMN phagocytosis, whereas 10 ng/ml showed low levels of PMN phagocytic capacity (Yang et al. Reference Yang, Ko, Kang, Song, Lee and Jeung2005). It was, therefore, assumed that the dose of recombinant TNF-α of 1 ng/ml is the most effective dose responsible for the phagocytic capacity of porcine PMN. Anti-rpTNF-α pAb completely abolished the enhanced phagocytic capacity of porcine PMN induced by the culture supernatant fraction from PBMC treated with t10c12-CLA. These findings indicate that t10c12-CLA stimulates porcine PBMC to produce TNF-α, which enhances the phagocytic capacity of PMN. Several reports are in agreement with these findings. The phagocytic capacity of neutrophils has been reported to be up regulated by TNF-α (Shalaby et al. Reference Shalaby, Aggarwal, Rinderknecht, Svedersky, Finkle and Palladino1985). Dietary t10c12-CLA has been reported to increase TNF-α mRNA levels 12-fold in isolated adipocytes and non-adipocytes (Tsuboyama-Kasaoka et al. Reference Tsuboyama-Kasaoka, Takahashi, Tanemura, Kim, Tange, Okuyama, Kasai, Ikemoto and Ezaki2000). The up regulation of TNF-α by t10c12-CLA has also been observed in mouse splenocytes (Kelley et al. Reference Kelley, Warren, Simon, Bartolini, Mackey and Erickson2002). Up regulation of TNF-α mRNA induced by t10c12-CLA has numerous beneficial effects including anti-adipogenesis (Park et al. Reference Park, Storkson, Albright, Liu and Pariza1999; Tsuboyama-Kasaoka et al. Reference Tsuboyama-Kasaoka, Takahashi, Tanemura, Kim, Tange, Okuyama, Kasai, Ikemoto and Ezaki2000), anti-carcinogenesis (Ip et al. Reference Ip, Masao-Welch, Shoemaker, Shea-Eaton and Ip1999), and immunostimulation (Kim et al. Reference Kim, Chung, Lee and Yang2003). The TNF-α signal is transmitted via crosslinking of the membrane-bound receptor molecules, TNF receptor (TNF-R)α and TNF-Rβ, on target cells (Vandenabeele et al. Reference Vandenabeele, Declercq, Beyaert and Fiers1995). After binding to its membrane-bound receptors, TNF-α mediates a wide range of effects such as the regulation of immune function, mediation of the inflammatory response and triggering of apoptosis (Hu, Reference Hu2003).
It has been reported that CLA decreases the synthesis of prostaglandins, in particular PGE2 (Li & Watkins, Reference Li and Watkins1998), and PGE2 has been shown to have profound down regulatory effects on various inflammatory cells (Levy et al. Reference Levy, Clish, Schmidt, Gronert and Serhan2001; Harris et al. Reference Harris, Padilla, Koumas, Ray and Phipps2002). PGE2 has been reported to regulate macrophage TNF-α production through negative feedback (Kunkel et al. Reference Kunkel, Wiggins, Chensue and Larrick1986; Ikegami et al. Reference Ikegami, Sugimoto, Segi, Katsuyama, Karahashi, Amano, Maruyama, Yamane, Tsuchiya and Ichikawa2001; Shinomiya et al. Reference Shinomiya, Naraba and Ueno2001). Therefore, one possible explanation for increased TNF-α expression in porcine PBMC treated with t10c12-CLA may be related to decreased PGE2 regulation of TNF-α. On the other hand, this effect of CLA may be also a general effect of n-6 PUFA since n-6 PUFA are known to be pro-inflammatory (Toborek et al. Reference Toborek, Barger, Mattson, Barve, McClain and Hennig1996). It has been suggested that perturbation of PUFA metabolism by CLA will have an impact on eicosanoid formation and metabolism, closely linked to the biological activities of CLA (Banni et al. Reference Banni, Petroni, Blasevich, Carta, Cordeddu, Murru, Melis, Mahon and Belury2004).
In the present study, PPARγ expression in porcine PBMC was increased by t10c12-CLA treatment. This finding is in accord with other reports that CLA increased PPARγ expression in tissue and cells such as skeletal muscle (Meadus et al. Reference Meadus, Maclnnis and Dugan2002), adipocytes (Evans et al. Reference Evans, Pariza, Park, Curtis, Kuebler and McIntosh2000) and macrophages (Yu et al. Reference Yu, Correll and Vanden Heuvel2002). When the binding affinity of t10c12-CLA to PPARγ was determined using a scintillation proximity assay, t10c12-CLA was found to be a ligand for PPARγ with an affinity ranging from 4·2 to 5·2 μm (Belury et al. Reference Belury, Moya-Camarena, Lu, Shi, Leesnitzer and Blanchard2002). It was suggested that the ability of CLA to induce PPARγ-responsive genes may be due to direct binding of CLA to the PPARγ as well as the binding of active metabolites of CLA produced via Δ6 desaturase. Therefore, it was thought that the actions of t10c12-CLA may be associated with increased levels of PPARγ protein and/or activation of PPARγ by downstream metabolites.
It has been reported that PPAR activators slightly increase TNF-α production in Kupffer cells (Nakatani et al. Reference Nakatani, Tsuboyama-Kasaoka, Takahashi, Miura and Ezaki2002). Increased PPARγ mRNA expression in the mesenteric tissue could lead to mesenteric fat hypertrophy, which could actively participate through the synthesis of TNF-α (Desreumaux et al. Reference Desreumaux, Ernst and Geboes1999). These observations support the idea that the activation of PPARγ by t10c12-CLA can regulate TNF-α gene transcription in porcine PBMC. The present results reveal that t10c12-CLA stimulates both PPARγ and TNF-α expression in porcine PBMC. We used a PPARγ antagonist, BADGE, which is a PPARγ ligand with a Kd(app) of 100 μm (Wright et al. Reference Wright, Clish, Mikami, Hauser, Yanagi, Hiramatsu, Serhan and Spiegelman2000), to elucidate the role of PPARγ on TNF-α expression in porcine PBMC induced by t10c12-CLA. BADGE negated the effect of CLA on TNF-α expression. This BADGE-induced decrease in TNF-α production by PBMC diminished the enhancement of PMN phagocytic capacity induced by the t10c12-CLA-stimulated PBMC culture supernatant fraction. These results suggest that the effects of t10c12-CLA on TNF-α production in porcine PBMC may be dependent on the PPARγ pathway. Therefore, it can be concluded that t10c12-CLA has an immunostimulating effect on porcine PMN phagocytic capacity, which is mediated by TNF-α production by PBMC via a PPARγ-dependent pathway.
Acknowledgements
The present study was supported by the National R & D Programme Grant of The Ministry of Science and Technology (M1-0417-06-0005), National Livestock Research Institute and the Ministry of Education and Human Resources Development (MOE), the Ministry of Commerce, Industry and Energy (MOCIE) and the Ministry of Labour (MOLAB) through the fostering project of the Laboratory of Excellency. In addition, the authors appreciate the graduate fellowship provided by the Ministry of Education through the BK21 programme. The authors also thank Dr Daehyun Chung, Department of Information and Statistics, Chungbuk National University, Republic of Korea, for statistically analysing the data.