Lipids are a major source of metabolic energy, a source of essential fatty acids (FA) which are precursors of important signalling molecules, the eicosanoids, and are components of biological membranes(Reference Vance and Vance1). Proper digestion and absorption of lipids in early life are therefore of vital importance. The digestion of lipids starts when fat is emulsified by bile acids to form micelles. Micelles increase the surface area of the lipids to make them available to digestive enzymes. Lipases, the common term for lipid-digesting enzymes, will then hydrolyse the ester bonds of the multitude of lipid classes including TAG, glycerophospholipids (PL) and wax esters. Some of the products from this digestion are NEFA, monoacylglycerol and lysophospholipids (lyso-PL), which are absorbed by the enterocytes together with bile(Reference Mansbach, Tso and Kuksis2).
Lipid digestion in fish and mammals is very similar. The principal difference lies in how TAG is hydrolysed. The primary intestinal neutral lipase in mammals is pancreatic lipase functioning together with co-lipase(Reference Brockman, Mansbach, Tso and Kuksis3), while in teleost fish, carboxyl ester lipase, also called bile salt-activated lipase (BAL), has the same role(Reference Lie and Lambertsen4, Reference Sæle, Nordgreen and Olsvik5). Digestion of PL is similar in mammals and fish, where the principal enzyme secreted is phospholipase A2 (PLA2) group IB(Reference Uematsu, Kitano and Morita6, Reference Iijima, Fujikawa and Tateishi7).
Mammals are born with deficient or immature lipase production in the pancreas(Reference Berton, Sebban-Kreuzer and Rouvellac8, Reference Miller and Lowe9). Similarly, teleost fish such as Atlantic cod (Gadus morhua) are hatched with a very low endogenous production of lipases(Reference Sæle, Nordgreen and Olsvik5, Reference Uematsu, Kitano and Morita6, Reference Darias, Murray and Gallant10). The most important contributors to the digestion of neutral lipids in suckling mice are pancreatic lipase-related protein 2 (PLRP) produced in the pancreas by the suckling pup and BAL, delivered in the milk. PLRP-2 is a protein with high structural similarities to pancreatic lipase. Atlantic cod has a very low pancreatic production of BAL when it starts ingesting food 3–5 d after hatching(Reference Sæle, Nordgreen and Olsvik5). Only one PLRP has been described in Atlantic cod, but it seems rather to be related to PLRP-1 than to PLRP-2 or pancreatic lipase(Reference Sæle, Nordgreen and Olsvik5). In mammals, PLRP-1 is also produced in the pancreas and excreted in the intestine but it does not have lipolytic capabilities; its function is currently unknown. It is thus uncertain whether the teleost version of PLRP has any digestive function.
Another similarity between the suckling mammal and teleost larvae is that their food contains active lipases. BAL has been described in the milk of several mammals(Reference Hernell, Blackberg and Chen11). Fish larvae feed on rotifers and copepods which contain neutral lipases(Reference Hoehne-Reitan, Kjorsvik and Reitan12). There are contradictory findings as to whether these exogenous lipases are of importance to the fish larvae(Reference Sæle, Nordgreen and Olsvik5, Reference Hoehne-Reitan, Kjorsvik and Reitan12), whereas in mammals, there is strong evidence that BAL in the mother's milk is an important contributor to lipid digestion(Reference Miller and Lowe9).
If exogenous lipases introduced with live prey are important for lipid digestion in teleost larvae, dietary pre-hydrolysed lipids might have the same effect. Experiments, in which teleost larvae were fed hydrolysed lipids with tube feeding, demonstrated a more efficient uptake of monoacylglycerol and diacylglycerol than intact TAG(Reference Mollan, Tonheim and Hamre13). On the other hand, it has been demonstrated in piglets that NEFA induce mucosal injury in the intestine and that the degree of injury increases with the carbon length of the FA(Reference Velasquez, Tso and Crissinger14). It is therefore important to clarify whether dietary NEFA fed over a longer period would increase lipid uptake, whether the type of hydrolysed lipid class was of importance and whether the hydrolysed lipid would induce injuries. Multiple diets were designed where either TAG or PL were hydrolysed to different degrees. The effects of dietary hydrolysed lipids over a longer period could thus be investigated.
Materials and methods
Experimental diets
For the present study, six diets were produced at the Institute of Marine Research, Austevoll, Norway, using an agglomeration technique. The dry components (Table 1) were thoroughly homogenised (Spar Mixer Fuji Paudal Company Limited), and then 20 % distilled water was added and mixed for 5 min. The mixture was transferred to an extruder (Multi Gran; Fuji Paudal Company Limited) with a 0·5 mm screen and screws turning at 20 rpm. The diet strings were transferred to a Marumeriser (Fuji Paudal Company Limited) and spun at 300 rpm for 15–20 s to produce small spherical pellets. The pellets were frozen in liquid N2, transferred to a centrifugal mill (Retsch ZM 200, Retsch) and centrifuged at 18 000 rpm, using a 1000 μm ring sieve. The pellets were then passed through different sieves (200, 315, 400 and 560 μm). The diets were stored at − 80°C until use.
Table 1 Diet composition of all diets

* Codrico, BAMA, Oslo.
† Rieber fish powder GVP, Rieber & Søn ASA, Bergen.
‡ Pepsin hydrolysed white fishmeal according to the method described by Kvale et al. (Reference Kvale, Harboe and Espe56)
§ Rieber squid meal, Rieber & Søn ASA, Bergen.
∥ As recommended by the National Research Council (1993). The vitamins and astaxanthin are from Hoffmann-La Roche Limited, Basel; the minerals from Merck, Darmstadt.
¶ 75 % cod-liver oil (Møllers Tran, Axellus AS) and 25 % EPAX 1050 TG oil (EPAX AS).
Preparation of lipids and addition to the basic diets
The six experimental diets had 15 % lipid, of which 40 % was hydrolysed or intact PL and 60 % was partly hydrolysed or intact TAG. The neutral lipase hydrolysed only 50 % of TAG, consequently there were no diets depleted of intact TAG. Diets 1 and 4 had no neutral lipid hydrolysation, resulting in approximately 40 % TAG of the total lipid. Diet 2 had 30 % TAG and diets 3 and 6, 20 % TAG. Hydrolysation of PL was almost complete in these diets where hydrolysed PL were introduced (diets 4, 5 and 6). Table 2 gives the different lipid class compositions of the diets.
Table 2 Lipid class composition in diets 1–6*

DAG, diacylglycerol; PL, glycerophospholipids; SM, sphingomyelin; CHOL, cholesterol; CL, cardiolipin.
* Numbers represent percentage of total lipid.
The phospholipid used in the diet was marine phospholipid (Phosphonorse®; Eximo). PL were hydrolysed with PLA2 from porcine pancreas (Sigma P0861, Sigma) according to the method described by Chung & Yang(Reference Chung and Yang15). The PL were dissolved in a 50:50 solution of ethyl acetate and sodium acetate buffer (50 mm, pH 5·6). PLA2 was added at 1 % of the total volume. The solution was kept at 30°C with continuous stirring for 6 h. The enzyme was deactivated at 80°C for 30 min. The solution was then dried in a rotavapor and frozen at − 20°C and freeze-dried to extract the rest of ethyl acetate and water. Later analysis showed that heating to 80°C was unfortunately not sufficient to deactivate the enzyme. Sodium acetate was also added to the diets containing non-hydrolysed PL. This was done to give an equal concentration of sodium acetate in all diets since this compound has the potential to saponify NEFA.
A mixture of 75 % cod-liver oil (Møllers Tran; Axellus AS) and 25 % EPAX 1050 TG oil (EPAX AS) was mixed to give the appropriate concentration of DHA and EPA in the final diets. The oil mixture was added to distilled water and a 1,3-specific lipase enzyme (Lipozyme TL 100 L; Novozymes) to the final concentration of 85 % lipid, 15 % water and 900 parts per million enzymes (Lipozyme TL 100 L; Novozymes). The solution was continuously stirred for 5 h at 55°C. The solution was heated to 80°C for 30 min to deactivate the enzymes. All oil mixtures had mixed tocopherols added (MTS-70; Archer Daniels Midland Company).
Analysis of the prepared diets
Crude protein (N × 6·25) was determined by total combustion using a nitrogen analyser (Leco FP-528, Leco). Lipid class composition was analysed by high-performance thin layer chromatography analysis (Iatron Laboratories, Inc.), using the method described by Henderson & Tocher(Reference Henderson, Tocher, Hamilton and Hamilton16). Total lipid was estimated as total FA by analysing FA composition in total lipids with GLC according to Lie & Lambertsen(Reference Lie and Lambertsen17), using 19 : 0 as an internal standard. DM content of the diets was determined gravimetrically after drying for 24 h at 104°C.
Biological parameters
The experiment was conducted in accordance with the Animal Welfare Act of 12 December 1974, no. 73, §§22 and 30, amended 19 June 2009. Cod eggs were collected from captive brood stock. Fertilised naturally spawned eggs were incubated and hatched in 70 litre tanks. The temperature in these units was similar to brood stock temperature, approximately 6°C. The majority of the eggs were hatched after 15 d and then transferred to the first feeding tanks (400 litres, with central aeration). The tanks had 24 h light and the larvae were fed rotifers (Brachionus plicatilis) in meals. Green water was made by using algae paste. Continuous feeding was initiated 3 d after transfer to the feeding tanks. Water temperature was gradually raised from 6 to 12°C over 6 d. At 30 d post-hatch (dph), the larvae were randomly distributed to eighteen 50 litre tanks, at the rate of 200 larvae in each tank. The larvae were only fed the trial diets from this point. The trial diets were fed in triplicate and the diets were assigned to the tanks in random order. The larvae were also in these tanks exposed to 24 h light. On each day, 10 ml of feed were put on a belt feeder. In the beginning, additional hand feeding was done. The tanks were cleaned daily and water flow was increased during the experiment so that water oxygen content did not decrease below 80 % saturation. Water temperature was maintained at 12 ± 0·5°C throughout the experiment.
Biological sampling
Larvae were sampled at 45 dph, and at the end of the experiment at 60 dph. All larvae were anaesthetised with metacaine (MS-222™; Norsk Medisinaldepot AS) and photographed, before euthanising with an overdose of metacaine. The larvae were pooled and snap-frozen in liquid N2 for lipid analysis, enzyme activity and RNA extractions. For histology, individual larvae were fixed in 4 % paraformaldehyde buffered in PBS (pH 7·4) overnight.
RNA extraction and quantitative PCR
Frozen larvae were homogenised on Quiazol (Qiagen) using a Precellys 24 (Bertin Technologies). Total RNA was extracted from the whole fish on a Bio Robot EZ1 using the EZ1 RNA Universal Tissue Kit with the RNase-free DNase Set (Qiagen), according to the manufacturer's instructions. The quality of RNA was assessed with the NanoDrop® ND-1000 UV–Vis Spectrophotometer (NanoDrop Technologies). For all total RNA samples, the optical density ratio at 260/280 nm ranged between 1·65 and 2·11.
Integrity of RNA was controlled in twelve randomly chosen samples out of the thirty-six using the Agilent 2100 Bioanalyzer and the RNA 6000 Nano LabChip® kit (Agilent Technologies). RNA integrity numbers(Reference Mueller, Hahnenberger and Dittmann18, Reference Imbeaud, Graudens and Boulanger19) were between 9·0 and 10.
RT-PCR were run in duplicates on a ninety-six-well plate. For efficiency calculations, a two-fold serial dilution of total RNA, mixed from all samples, ranging from 1000 to 31 ng/μl, was run in triplicates. All reactions were synthesised with a 500 ng total RNA input. Each plate included a no-template control and a RT control (a reaction without an RT enzyme). Plates were run on a GeneAmp PCR 9700 (Applied Biosystems) using the TaqMan Reverse Transcription Reagent containing Multiscribe Reverse transcriptase (833·5 nkat/μl (50 U/μl); Applied Biosystems).
Reverse transcription was performed at 48°C for 60 min by using a oligo dT primers (2·5 μm) for all genes in 30 μl total volume. The final concentrations of other chemicals in each RT reaction were as follows: MgCl2 (5·5 mm), deoxyribonucleotide triphosphate (500 mm of each), 10X TaqMan RT buffer (1 × ), RNase inhibitor (6·668 nkat/μl (0·4 U/μl)) and multiscribe RT (27·8389 nkat/μl (1·67 U/μl)).
From each RT reaction, 2 μl complementary DNA were pipetted in duplicates to 384-well plates, followed by 18 μl consisting of 7·6 μl double-distilled water, 10 μl SYBR Green PCR Master Mix (Applied Biosystems) and 0·2 μl of each primer (50 μm forward and reverse). Pipetting was done using a robot Biomek 3000 (Beckman Coulter). Real-time PCR was run on the LightCycler® 480 Real-Time PCR System (Roche Applied Sciences), with a 5 min activation and a denaturing step at 95°C, followed by forty cycles of a 15 s denaturing step at 95°C, a 60 s annealing step and a 30 s synthesis step at 72°C. The annealing temperature was 60°C for all primer pairs. Results were calculated as the arithmetic mean. Ribosomal protein L37, ubiquitin and elongation factor 1A were selected as reference genes based on the study of Sæle et al. (Reference Sæle, Nordgreen and Hamre20). The geNorm visual basic for applications applet for Microsoft Excel was used to determine a normalisation factor based on the reference genes(Reference Vandesompele, De Preter and Pattyn21). Primer sequences, product size and PCR efficiency for each quantitative PCR assay are given together with the corresponding accession number in Table 3.
Table 3 Gene names, symbols, accession number, primer sequences for SYBR Green assays, SYBR Green assay product size and PCR assay efficiency for all the genes analysed

Lipase activity
Whole larvae and dissected gastrointestinal tracts were homogenised in four times the sample wet weight of PBS with an Ultra–Turax® 20 000 (UpM, Janke u. Kunkel KG) followed by a second homogenisation in a ball mill (Retsch® MM301, Retsch), for 2 × 2 min (thirty shakes/s). Homogenates were centrifuged at 10 000 g for 15 min at 4°C and supernatants used for enzyme activity were measured for total protein with the BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
Neutral lipase activity measurements were based on Murray et al. (Reference Murray, Gallant and Perez-Casanova22), with modifications according to Sæle et al. (Reference Sæle, Nordgreen and Olsvik5). The assay uses 0·4 mm-4-nitrophenyl myristate, which serves as a substrate for most neutral lipases, in 25 mm-ammonium bicarbonate, 0·125 m-sodium cholate, 37·5 mm-NaCl, pH 7·8, at 25°C. Assays were done in ninety-six-well plates that were read every 15 s for 30 min at 404 nm with a microplate reader (iEMS Reader MF; Labsystems Oy).
The Cayman Chemical secretory PLA2 assay kit (item no. 765001; Cayman Chemical Company) was used for enzyme activity calculations. The assay uses the 1,2-dithio analogue of diheptanoyl phosphatidylcholine (PC), which serves as a substrate for most PLA2 with the exception of cytosolic PLA2(Reference Reynolds, Hughes and Dennis23, Reference Hendrickson, Hendrickson and Dybvig24). Upon hydrolysis of the thio ester bond at the sn-2 position by PLA2, free thiols are detected using 5,5′-dithio-bis-(2-nitrobenzoic acid). Plates were read every 15 s for 30 min at 414 nm with a microplate reader (iEMS Reader MF).
Reduced glutathione and oxidised glutathione
The microplate assay for reduced glutathione/oxidised glutathione (Oxford Biomedical Research) was used according to the manufacturer's instructions. Larvae (four to five from 45 dph or two to three from 60 dph) were pooled before homogenisation. The pyridine derivate, serving as a thiol-scavenging reagent, was added before homogenisation of larvae in the assay buffer.
Thiobarbituric acid-reactive substances
This analysis was performed according to Hamre et al. (Reference Hamre, Næss and Espe25). In short, lipid was removed from samples with the Bligh and Dyer extraction. An aliquot from the methanol/water phase was added to thiobarbituric acid in excess; when heated, malondialdehyde reacts with thiobarbituric acid and forms a coloured complex. The reaction is measured with a spectrophotometer at 532 nm and quantified by comparison with a standard.
Histology
Fixed specimens were embedded in resin (Technovit 7100). Then, 2 μm sections in the longitudinal direction were made of the whole specimens and stained with borax-buffered toluidine blue. The sections were photographed using an Olympus BX51 microscope (Olympus) with a Nikon DS Fi1 camera (Nikon Instruments).
Statistical analysis
Potential differences were tested with one-way ANOVA. Fisher's least significant difference post hoc test was used to identify significant differences between group means. Differences and effects were considered significant at P< 0·05, and all tests and differences were annotated with unlike letters in figures. ANOVA and post hoc analyses were performed on Statistica 10.0 (StatSoft, Inc.). Data are presented as means and standard deviations.
Results
Diets and fish larvae
All diets contained equal concentrations of the analysed nutrients; with 44·1 (sd 0·3) % protein, 16·9 (sd 0·8) % lipid and 38·9 (sd 1·0) % carbohydrate in DM and ash, and had 70·1 (sd 0·6) % DM. There was no difference in thiobarbituric acid-reactive substances levels between the diets, with an average level of 46·7 (sd 8·4) nmol/g wet weight. Due to the immediate change from rotifers to the formulated diets, there was a period of low feed intake in all diet groups that increased mortality and delayed growth. There was no observed cannibalism during the trial. This abrupt diet switch without co-feeding was done to avoid a situation where the fish larvae could selectively feed on rotifers, thus introducing the uncertainty about different preferences between the diets. The standard length of the larvae was 8·3 (sd 0·5) mm at the start of the trial. During the following 15 d, it increased to an average of 9·6 (sd 1·5) mm to reach 15·7 (sd 3·2) mm at the end of the trial. Growth data demonstrate that energy accumulation was the same in all diet groups. There were no differences in lengthwise growth between the treatments, but fish given the control diet (diet 1) were heavier than the other groups at mid-trial. However, this difference disappeared at the end of the trial (Table 4). There were no larvae left in the groups fed diet 6 at the final sampling due to high mortality. Mortality increased with increasing levels of NEFA in the diets with a correlation of R 2 0·78 (Fig. 1). The correlation between lyso-PL and mortality was R 2 0·59.
Table 4 Growth of fish larvae fed diets 1–6, after 15 d (45 d post-hatch (dph)) and 30 d (60 dph) (Mean values and standard deviations, n 20)
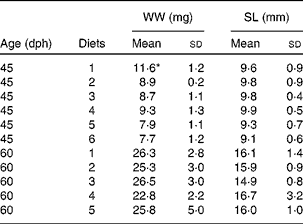
WW, wet weight; SL, standard length.
* Mean value was significantly different from those of the other diet groups (P< 0·05; ANOVA).

Fig. 1 Correlation between NEFA in the diets and cumulative mortality per experimental unit (P< 0·000; R 2 0·8, n 18).
Lipids
There were differences in lipid levels between larvae fed the different diets after 15 d (45 dph), but these differences had disappeared after 30 d (60 dph). The 45 dph larvae fed diet 2 had higher neutral lipids (12·0 (sd 0·4) mg/g wet weight) compared with those fed diets 1, 3 and 4 (9·6 (sd 0·9) mg/g wet weight). Larvae fed diet 5 had lowest neutral lipids with a content of 7·1 (sd 0·9) mg/g wet weight (P< 0·05, one-way ANOVA; Fig. 2). At the end of the trial, there were no differences in lipid levels between the groups (average for all groups: 15·2 (sd 2·5) mg/g dry weight). Polar lipid levels were 22·7 (sd 1·8) mg/g wet weight for diet groups 1, 2, 3 and 4 and decreased to 7·1 (sd 0·9) mg/g wet weight in larvae fed diet 5 (P< 0·05, one-way ANOVA; Fig. 2) at 45 dph. At the end of the trial, the polar lipid level increased to 25·7 (sd 3·3) mg/g wet weight for larvae fed diets 1–5. Due to low survival in larvae fed diet 6, only larvae from one tank were analysed at day 15. These larvae contained 16·82 mg/g wet weight of polar lipids and 8·34 mg/g wet weight of neutral lipids. The most abundant PL, PC and phosphatidylethanolamine (PE), showed the same quantity distribution between the diet groups as seen in the total polar lipids, as did cardiolipin (Fig. 2). However, phosphatidylserine (PS) and phosphatidylinositol (PI) were lower in the diet 3 group than the diet 1, 2 and 4 groups (Fig. 2). TAG was higher in the diet 2 group than in the diet 1, 3 and 4 groups, whereas the diet 5 group was lowest (Fig. 2).

Fig. 2 Lipid class levels (mg/g wet weight) in fish larvae at mid-trial. The inserts show the lipid class level in the diets. Values are means, with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA). Fish given diet 6 were not included in the statistical analysis since only one unit could be analysed. PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; CL, cardiolipin.
Enzyme activity
Neutral lipase activity in whole fish was 0·113 (sd 0·032) μmol/min per g at 45 dph and 0·132 (sd 0·019) μmol/min per g at 60 dph. PLA2 activity was 0·037 (sd 0·014) μmol/min per g at 45 dph and 0·029 (sd 0·012) μmol/min per g at 60 dph. There were no effects of the diets on the enzymatic activity.
Lipid metabolism and oxidative stress
Larval mRNA levels of PPARβ and cytochrome P450 4 and 1A (CYP4 and CYP1A) did not vary significantly between the diet groups at mid-trial or at the end of the trial. Farnesoid X receptor (FXR) mRNA was stable between the diet groups at mid-trial but at the end of the experiment, mRNA levels were elevated in larvae fed diets 2 and 3 compared with diets 1, 4 and 5. A similar pattern was seen in mRNA levels of stearoyl-CoA desaturase (SCD; Table 5).
Table 5 Overview of the genes analysed, their function and the P value of quantitative PCR analysis (one way-ANOVA)

GPX, glutathione peroxidase; GSSG, oxidised glutathione; GSH, reduced glutathione; ROS, reactive oxygen species.
There were no differences in the levels of reduced glutathione or oxidised glutathione in larvae fed the different diets. Reduced glutathione was 474 (sd 73) μm in 45 dph larvae and 599·9 (sd 108·2) μm in 60 dph larvae. Oxidised glutathione was 2·2 (sd 0·8) and 2·5 (sd 1·8) μm in 45 and 60 dph larvae, respectively. mRNA levels of thioredoxin reductase (TXNRD3), CuZn superoxide dismutases (CuZn SOD), Mn superoxide dismutases (Mn SOD), glutathione peroxidase 1 and 4 (GPx 1 and 4), glutathione reductase (GSR), catalase (CAT), thioredoxin (TXN), metallothionein (MT), NADPH oxidase 1 (NOX1), glutaredoxin 3 (GLRX3), glutamate-cysteine ligase (GCLC) or cell division cycle and apoptosis regulator 1 (CCAR1) were similar in all diet groups (Table 5), both at 45 and 60 dph. However, glutathione peroxidase 3 (GPx 3) mRNA was lower in larvae fed diet 5 compared with those fed diet 3 at 60 dph (Fig. 3).

Fig. 3 Mean normalised expression (MNE) of the genes that responded to the trial diets after 30 d. (A) Glutathione peroxidase 3 (GPx3), (B) farnesoid X receptor (FXR) and (C) stearoyl-CoA desaturase (SCD). Values are means, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; ANOVA).
Lipid-induced injury on enterocytes
After 15 d on the trial diets, the presence of lipid vacuoles (droplets) in the enterocytes was similar in fish given diets 1–3. In fish larvae fed diets 4 and 5, lipid vacuoles had increased in numbers and started to merge. In fish given diet 6, the enterocytes were completely destroyed (Fig. 4). At the end of the trial, severe injury on the intestinal epithelium, such as rupture of the cell membrane of the enterocytes, was observed in fish larvae given all the different diets except the control diet (Fig. 4). The severity of intestinal injuries was amplified with the increasing amount of NEFA in the diets.

Fig. 4 Histology of the intestinal mucosa at mid-trial (45 d post-hatch (dph)) and at the end of the trial (60 dph). 1, Intestinal lumen; 2, mucosal goblet cells; 3, food particles in the lumen; 4, lipid droplets in enterocytes; 5, lysed epithelium. Scale bar, 50 μm (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Discussion
The quantity of the PL classes (PC, PE, PS, PI and cardiolipin) in fish fed diets with intact PL was all similar. In fish fed diets with hydrolysed PL, only the diet group with intact TAG was able to maintain the PL level at the same level as the diet groups being fed intact PL. There was a general trend that when fed hydrolysed PL, the larvae were dependent on intact TAG to maintain the PL levels. This means that NEFA in the diet was probably not available for the de novo synthesis of PL or the remodelling of lyso-PL. Our laboratory experiment has previously shown that when cod larvae were fed diets in which part of the dietary lipids were NEFA, nearly all of the NEFA was catabolised, thus not integrated into lipids to be stored in the body(Reference Hamre, Lukram and Ronnestad26). It has also been demonstrated in yeast that intact TAG, and not NEFA, is a vital source of FA in PL metabolism. In yeast strains without the ability to hydrolyse TAG, PL synthesis was severely affected(Reference Rajakumari, Rajasekharan and Daum27). We have here demonstrated that the lack of dietary intact TAG affects the synthesis (de novo or remodelling) of PL, PC and PE. This might explain the lack of a positive effect on growth in larvae fed the low dietary levels of NEFA (e.g. diet 2).
Lipids are an important source of energy for cod larvae; 100 % of absorbed lipids are catabolised until a threshold level of retention is reached. This threshold is lower for polar lipids than for neutral lipids(Reference Hamre, Lukram and Ronnestad26). Here we demonstrate that TAG retention in larvae is maintained in larvae given hydrolysed TAG as long as the PL fraction is intact. This implies that larvae maintain a minimum level of neutral lipids at the cost of other lipid classes which are important for growth. However, this is not reflected in PC or PE levels, but might explain the decrease in PS and PI in fish given diet 3.
NEFA has cytotoxic effects(Reference Listenberger, Han and Lewis28). This is avoided by incorporation into TAG for storage or into PL of cellular membranes. Another way of dealing with the hazard is degradation via α-, β- and ω-oxidative pathways(Reference Antony and Landau29). When β-oxidation is not sufficient for clearing the NEFA pool, the ω-oxidative pathway becomes important(Reference Wanders, Komen and Kemp30). The Cyp4 family is associated with the ω-oxidative pathway(Reference Adas, Salaun and Berthou31). However, this system seemed not to be affected when larvae were fed diets with increasing levels of NEFA. Other genes associated with toxic levels of NEFA and FA homeostasis such as SCD (Reference Ntambi32, Reference Flowers and Ntambi33) and FXR (Reference Trauner, Claudel and Fickert34, Reference Wang, Chen and Moore35) were up-regulated in larvae fed diets with partly hydrolysed TAG and intact PL. Fish given diets in which PL was hydrolysed did not induce a regulation different from the control diet. It is uncertain why the diets with the most NEFA did not lead to an up-regulation of FA homeostasis regulating genes while the moderately hydrolysed diets did. A possibility is that the intestinal epithelium was so damaged by the diets with hydrolysed PL that NEFA uptake was severely compromised at this point.
Cahu et al. (Reference Cahu, Infante and Barbosa36) demonstrated that the ratio of TAG and PL modulated the gene expression of BAL as well as the total neutral lipase enzymatic activity in sea bass (Dicentrarchus labrax) larvae. Genetic and enzymatic expression was modulated by dietary lipids as well. The diets of the present study varied in intact TAG:PL ratios, but this had no effect on the enzymatic activity of neutral lipases or phospholipases. This is similar to the findings of Morais et al. (Reference Morais, Cahu and Zambonino-Infante37) who did not find any effects of dietary lipid levels on mRNA levels or the activity of neutral lipases in sea bass (D. labrax) larvae. A possible explanation of the apparent inconsistency of these studies is the large difference in larval growth in the study of Cahu et al. (Reference Cahu, Infante and Barbosa36), compared with the study of Morais et al. (Reference Morais, Cahu and Zambonino-Infante37) and the present one. When fish larvae are analysed according to age and not size, different developmental stages may be compared(Reference Saele and Pittman38).
Lipid-induced injury on the intestinal epithelium in fish has previously been reported in fish fed diets high in TAG and low in PL(Reference Fontagne, Geurden and Escaffre39, Reference Deplano, Connes and Diaz40). These injuries were attributed to steatosis due to the accumulation of lipid droplets in the enterocytes. The accumulation is thought to be caused by the need of dietary PL to assemble lipids in chylomicrons and VLDL for lipid transport from the intestine to peripheral tissues. Lipid accumulation in intestinal tissue due to high dietary TAG:PL ratios has been described in a wide range of fish species(Reference Fontagne, Geurden and Escaffre39–Reference Sarasquete, Polo and Yufera47). The injuries found in the present study were not caused by a high TAG:PL ratio, but bear the same characteristics. If intact dietary PL were an important factor in injuries, and consequent mortality, in the present study, there should have been a marked difference in injury and mortality between the diets with intact PL and those with hydrolysed PL, e.g. fish fed diet 3 v. fish fed diet 4. This was not the case. Instead, the degree of intestinal injury and mortality was correlated with the amount of NEFA in the diets. However, the toxic effect of NEFA did not affect growth measurements during the trial period. This indicates that dietary NEFA had a more toxic effect on larvae with poor growth than the more fit larvae. Similar findings have been found in red sea bream (Pagrus major) larvae where different groups exposed to increasing levels of Zn had increasing mortality from 0 to almost 100 %. The increasing Zn exposure did not influence the length of the surviving fish larvae(Reference Huang, Cao and Shan48).
Lyso-PL may perturb membrane homeostasis and increase membrane fluidity and permeability(Reference Farooqui and Horrocks49). Sawai et al. (Reference Sawai, Drongowski and Lampman50) demonstrated that lyso-PC produced by PLA2 activity increased bacterial translocation as well as decreased transepithelial electrical resistance over the membrane. This did not occur when PL species other than PC were hydrolysed. NEFA alone did not affect bacterial translocation, but did decrease transepithelial electrical resistance in Caco-2 cells. The present results do not point towards lyso-PL as being the cause of death, indicating that membrane translocation of bacteria in the enterocytes has not been a problem. Decreased transepithelial electrical resistance, however, would be a challenge for the animal's osmoregulation.
The accumulative effect of dietary NEFA on the intestinal tissue of Atlantic cod larvae was similar to that found in mice given ricinoleic acid (the main toxic component in castor oil). In these mice, ricinoleic acid led to the exfoliation of enterocytes, exposing the subepithelial tissue to bacteria(Reference Morehouse, Specian and Stewart51). Similar exfoliation or degradation of enterocytes was observed in the present trial, in fish larvae fed diets with 2·7 % NEFA (diet 3) or more. It is thus possible that mortality was due to infections and/or osmotic stress due to the exposure of the subepithelial tissue.
Degradation of the intestinal epithelial tissue is therefore probably caused by NEFA. Lapre et al. (Reference Lapre, Termont and Groen52) demonstrated that NEFA with a carbon chain length of ten (C10) or more had a lytic effect on cells. Unsaturated FA were more lytic than SFA, but there were no differences between 18 : 1 and 18 : 2. The authors also demonstrated that the cytolytic effect of NEFA on Caco-2 cells was similar to the effect on erythrocytes, indicating that the lytic effect is not cell dependent. Other studies have shown endotheloid cells to be more resistant to NEFA than muscle cells. Endotheloid cells were lysed by 16 : 0 and longer FA after 36 h exposure, whereas muscle cells were also lysed by 14 : 0(Reference Wenzel and Hale53). When exposed to longer unsaturated FA (18 : 1, 18 : 2, 18 : 3 and 20 : 4), endotheloid cells were lysed by 20 : 4 after 4 h but not by shorter FA. Muscle cells, on the other hand, were vulnerable to both 18 : 3 and 20 : 4 after 2–4 h exposure(Reference Wenzel and Hale54). Considering these effects on cells in culture, it is quite probable that chronic exposure to NEFA through the diet was a strain on the epithelia of the gut. Even though enterocytes are naturally exposed to NEFA during any meal, the exposure is controlled by the rate of hydrolysis by the digestive enzymes BAL and PLA2. The concentration of NEFA in the unstirred water layer is therefore manageable for the cell membrane to absorb (Fig. 5(A)). If the concentration of NEFA in the unstirred water layer is increased, the passive association of NEFA with the plasma membrane will increase accordingly. This may lead to destabilisation of the membrane as illustrated in Fig. 5(B).

Fig. 5 Proposed cause of membrane disruption and subsequent exfoliation of enterocytes. (A) Transbilayer diffusion (flip-flop): 1, NEFA enters the outer leaflet of the cell membrane; 2, NEFA is transferred to the inner leaflet; 3, NEFA enters the cytosol bound to fatty acid-binding protein. (B) Overload of NEFA in the bilayer: 1, a large number of NEFA enter the cell membrane; 2, the membrane is destabilised and the cell lyses (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
There are very few in vivo studies on the effect of dietary NEFA; however, Velasquez et al. (Reference Velasquez, Tso and Crissinger14) found a similar effect in newborn piglet intestines to those described in Caco-2 cells and enterocytes. 8 : 0 FA gave fewer injuries than 10 : 0, 12 : 0 and 16 : 0. 18 : 1 proved to be more harmful than shorter FA but not 18 : 3. They also found that 1-d-old piglets were more sensitive to lipid-induced mucosal injury than 1-month-old piglets. This might also be true for fish such as Atlantic cod. For example, there are reports of experiments where adult Atlantic cod have been fed diets with up to 3·8 % NEFA without increased mortality(Reference Lie, Lied and Lambertsen55).
In summary, we demonstrate the toxic effects of dietary NEFA on Atlantic cod larvae. Despite previous findings indicating a positive effect of dietary NEFA over periods of time counted in hours, we here demonstrate that over periods of days (15–30), dietary NEFA appear to be chronically toxic. Perhaps most surprisingly is the lack of a positive effect at low levels of dietary NEFA. The toxicity is probably not due to oxidative stress but to the lytic effect of NEFA on the intestinal epithelium.
Acknowledgements
This study is a part of the project financed by the Research Council of Norway: Ontogeny of lipid digestion and effects of feeding pre-digested lipids to Atlantic cod (G. morhua) larvae 179016/S40. We thank Samuel James Penglase for painstaking correction of the manuscript and for the valuable inputs. The contributions of the authors are as follows: A. N. and J. I. H. were responsible for the production of the experimental diets and lipid analysis. P. A. O. identified the genes analysed and designed the quantitative PCR primers. Ø. S. was the principal author, and performed the mRNA, protein, enzyme and histology analysis. T. H. ran the experiment. K. H. was the project leader and planned the study. All authors contributed to the writing of the manuscript. There are no conflicts of interest.












