Health and nutrition are intimately linked. This raises the issue of quantifying and balancing the constituents associated with a given food. Such an analysis requires knowledge of those constituents’ concentrations that are effectively absorbed by the human body( Reference Cardoso, Afonso and Lourenço 1 ). In order to achieve this purpose, one must take into account that foods are typically subjected to further culinary treatment before ingestion and that the level of a nutrient in a food that is eaten may be quite different from the bioaccessible level – that is, the component concentration that is released from the food matrix into the intestinal lumen after digestion( Reference Cardoso, Afonso and Lourenço 1 ).
According to the literature( Reference Alipour, Shabanpoor and Shabani 2 , Reference Kong, Tang and Rasco 3 ), the culinary procedures used to prepare fish are associated with important physical and chemical changes – namely, moisture variation, alterations in the ability to retain moisture, discolouration, building up of particular aromas and textural transformations. These latter changes have been ascribed to protein denaturation( Reference Harris and Shorthose 4 ). Moreover, the influence of cooking upon bioaccessibility and the characteristics of the bioaccessible part are not well known. It should be remarked that, for any particular food nutrient or contaminant, bioaccessibility is a gauge of its maximal bioavailability – the portion of that nutrient/contaminant that is able to achieve systemic circulation( Reference Cardoso, Afonso and Lourenço 1 ). In addition, depending on numerous factors such as type and processing of food, the studied food constituent may be more or less bioavailable( Reference Afonso, Costa and Cardoso 5 – Reference Wienk, Marx and Beynen 7 ).
Recent experimental studies have been carried out with the aim of finding a suitable in vitro digestion model that is able to simulate in a realistic way the human digestive system( Reference Cardoso, Afonso and Lourenço 1 ). Such a challenge is compounded with extra technical difficulties when the targeted food constituent is also present in the substances used for the simulation of digestion, as in the case of lipids, which are found in the bile( Reference Afonso, Costa and Cardoso 5 ). Indeed, there are only a few studies on lipid bioaccessibility( Reference Cardoso, Afonso and Lourenço 1 , Reference Costa, Afonso and Cardoso 8 ). Besides, lipid class distribution of fatty acids (FA) may influence the bioaccessibility of particular FA, thereby adding another layer of complexity to the issue. For instance, it has been observed in in vivo studies with humans that FA bound to TAG are more bioavailable than FA bound to ethyl esters EE)( Reference Dyerberg, Madsen and Møller 9 , Reference Neubronner, Schuchardt and Kressel 10 ). These EE can be found in fish oil supplements, but not in the large majority of foods.
On the other hand, fish contain significant amounts of n-3 PUFA – namely, EPA and DHA – which are associated with decreased morbidity and mortality from CVD and other diseases as well as with fetal development( Reference Simopoulos 11 ). This justifies a greater attention to the issue of FA bioaccessibility in seafood. In particular, in the general context of increasing worldwide demand for fish products and the decline in wild marine resources, farmed fish has gained much importance( 12 ), with gilthead seabream (Sparus aurata) being one of the most valued and consumed seafood( 12 ).
Therefore, seabream was selected for this case study in order to achieve a deeper understanding of FA bioaccessibility and its dependence on the changes occurring during a more drastic culinary treatment (grilling) and on the particular lipid class distribution of each FA.
Methods
Fish samples
The sampling and the cooking methods used are described in detail by Costa et al.( Reference Costa, Afonso and Bandarra 13 ). In brief, gilthead seabream (S. aurata) were supplied by a Portuguese fish farm (average weight: 430 (sd 22) g; average length: 30 (sd 1) cm). Fish were gutted, beheaded, washed and sliced in half. One fillet was used for the culinary treatment and the other was used as the corresponding raw sample.
In each traditional cooking treatment, nine individuals were included. The grilling process was performed as described by Costa et al.( Reference Costa, Afonso and Bandarra 13 ). After cooking, the skin and bones were removed, and the collected edible part was homogenised. This material was separated into two sub-samples: one was frozen and stored at −80°C, and the other was frozen at −20°C, freeze-dried and then stored at −80°C until further analysis.
Bioaccessibility
Raw and grilled seabream samples were used to determine the bioaccessibility of fat and its components. Grilling is an ideal process for studying the influence of culinary treatments, as it belongs to the group of harsh cooking methods (from the point of view of its impact on the concentrations of fish constituents)( Reference Trevisan, Lima and Sampaio 14 ).
For this purpose, seabream samples were subjected to a previously perfected in vitro digestive model that simulates the regular physiology of the human digestive system with typical meals and quantities of food( Reference Afonso, Costa and Cardoso 5 ). There were no changes to the described methodology.
The bioaccessibility (%) of lipid, FA and protein was calculated in the same way( Reference Afonso, Costa and Cardoso 5 ), with the concentration of the nutrient found in the enzyme solution subtracted from the final result.
Analyses
Moisture, protein and lipid contents
Moisture content was determined according to Association of Analytical Communities methods( 15 ) by drying samples at 105°C. The protein level was quantified using a combustion method of analysis with the FP-528 DSP LECO nitrogen analyzer (LECO) calibrated with EDTA according to the Dumas method( Reference Saint-Denis and Goupy 16 ). Total fat was determined following the Smedes extraction method( Reference Smedes 17 ) using propan-2-ol and cyclohexane as solvents.
Lipid extraction
Extraction of fat from raw and grilled seabream samples was carried out using the Smedes technique( Reference Smedes 17 ). On the other hand, a different method was applied for lipid extraction from the bioaccessible portion, because fat is released from the food matrix into the bioaccessible phase as a consequence of several steps involved in the in vitro model. This procedure was identical to that previously described for salmon( Reference Costa, Afonso and Cardoso 8 ).
Lipid class determination
The amount of different lipid classes was estimated by TLC following an optimised technique( Reference Bandarra, Batista and Nunes 18 ). For elution, a mixture of hexane–diethyl ether–acetic acid (50:50:2, v/v/v) and a 0·25-mm silica gel G plate were combined. The position of each lipid class was confirmed through elution of a mixture of standards (Sigma Chemical Co.). The relative importance of the various lipid classes was assessed and quantified on the basis of image analysis with a GS-800 densitometer and version 4.5.2 of Quantity One 1-D Analysis software from Bio-Rad.
Separation of lipid classes and fatty acid profile
The different lipid classes were separated for FA analysis according to previously described procedures involving solid-phase extraction and preparative TLC( Reference Costa, Afonso and Cardoso 8 ). Main fat classes, TAG, diacylglycerol (DAG), monoacylglycerol (MAG), NEFA and phospholipids (PL) were confirmed by sigma standards.
The FA profile was determined in seabream before and after digestion (bioaccessible portion) by acid-catalysed transesterification, as described by Bandarra et al.( Reference Bandarra, Batista and Nunes 19 ). Results in mg/100 g were attained through the peak area ratio and the lipid adjustment coefficients proposed by Weihrauch et al.( Reference Weihrauch, Posati and Anderson 20 ).
Statistics
All data were analysed using STATISTICA 6 software (StatSoft Inc.). One-way ANOVA was used to determine significant differences (P<0·05) between raw and grilled seabream, and a factorial ANOVA was applied to determine significant differences (P<0·05) among lipid classes and between FA (before and after digestion), followed by a multiple comparison test (Tukey’s honest significant difference; HSD). When data could not satisfy normal distribution and homoscedasticity requirements, differences were analysed with non-parametric ANOVA (Kruskal–Wallis) followed by non-parametric multiple comparisons test (or Mann–Whitney)( Reference Zar 21 ).
Results and discussion
Effect of culinary treatment
Lipid fraction
The lipid content of seabream edible muscles was analysed in raw and grilled fish (Table 1). The lipid content was reduced because of oil drip losses during grilling. The main lipid classes in raw and grilled seabream before digestion are shown in Table 2 and were similar. Seabream has a large share of storage lipids, mainly found as TAG, as in other fish species( Reference Stubhaug, Tocher and Bell 22 ). Indeed, the TAG were always over 85 % of the total fat content, whereas structural lipids, mainly PL, comprised 4–5 % of the total fat content. The NEFA fraction was also in this range.
Table 1 Protein and lipid contents (g/100 g) as well as the fatty acid profile (mg/100 g) of raw and grilled gilthead seabream (Averages and standard deviations)
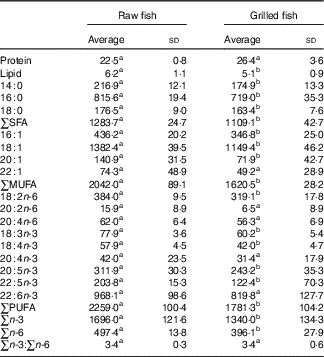
a,b Values within a row with unlike superscript letters were significantly different (P<0·05).
Table 2 Distribution of main lipid classes (%) in raw and grilled gilthead seabream before (initial) and after digestion (bioaccessible)
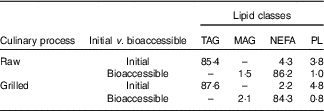
MAG, monoacylglycerol; PL, phospholipids.
These results are compatible with values reported in previous studies in gilthead seabream( Reference Valente, Cornet and Donnay-Moreno 23 – Reference Nasopoulou, Karantonis and Zabetakis 25 ) and in salmon( Reference Costa, Afonso and Cardoso 8 , Reference Aursand, Bleivik and Rainuzzo 26 ). TAG are always the most abundant class in fish containing more than 5 % fat, comprising 90 % of the total lipid content( Reference Costa, Afonso and Cardoso 8 ). On the other hand, NEFA were not detected in salmon( Reference Costa, Afonso and Cardoso 8 ). Nevertheless, their content in seabream was low, as expected in a non-hydrolysed fish fat fraction. Moreover, given the nature of grilling as a culinary treatment( Reference Costa, Afonso and Bandarra 13 ), no important change in lipid class composition was within expectations.
Fatty acid profile
The FA profiles of the raw and grilled seabream muscles are displayed in Table 1, and the specific profiles of each lipid class are shown in Table 3.
Table 3 Fatty acid profile (%) of the main lipid classes of raw and grilled gilthead seabream (Averages and standard deviations)
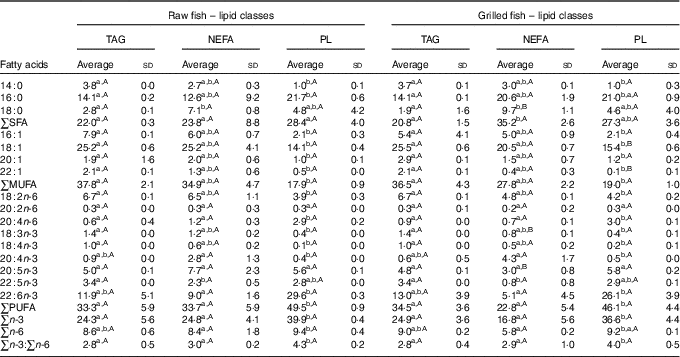
PL, phospholipids.
a,b For each culinary treatment, different unlike lowercase superscript letters within a row correspond to statistical differences (P<0·05). A,B For each lipid class, different unlike uppercase superscript letters within a row correspond to statistical differences (P<0·05).
For raw and grilled fish, PUFA were the most abundant FA group, followed by MUFA and SFA. This distribution was richer in PUFA than that of other farmed fish species such as salmon( Reference Costa, Afonso and Cardoso 8 ), but within the PUFA range found in other seabream studies( Reference Wassef, Saleh and El-Hady 27 ). The n-3 PUFA represented a much larger share of the total compared with the n-6 PUFA, yielding a n-3:n-6 ratio higher than 1 – 3·4 (sd 0·3) and 3·4 (sd 0·6) for raw and grilled fish, respectively. This ratio was also higher than that calculated for gilthead seabream fed a reference diet in another study (see the Fish samples section)( Reference Wassef, Saleh and El-Hady 27 ), and even for seabream in an integrated aquaculture system (see the Statistics section)( Reference Valente, Cornet and Donnay-Moreno 23 ). The most abundant FA in fat extracted from seabream was oleic acid (18 : 1), which contributed almost to a fourth of the total lipid content. This finding is also in accordance with those of a previous study( Reference Wassef, Saleh and El-Hady 27 ), showing that oleic acid level was the highest (39 % of total FA) among all in farmed seabream muscle. The other FA had the following abundance order: DHA (22 :6n-3)>palmitic acid (16 : 0)>palmitoleic acid (16 : 1)>linoleic acid (18 : 2n-6)>EPA (20 : 5n-3), all exceeding 200 mg/100 g in both raw and grilled fish. These results are significantly different from those reported for other farmed fish( Reference Valente, Cornet and Donnay-Moreno 23 ), as palmitic and linoleic acids were more abundant than DHA in seabream reared in intensive and semi-intensive systems. Furthermore, eicosenoic acid (20 : 1) content was similar to that of the current study’s seabream( Reference Valente, Cornet and Donnay-Moreno 23 ). These differences are more related to the particular feed used in rearing farmed seabream.
The statistical differences between FA concentrations in raw and grilled seabream muscles could be ascribed to fat variations with culinary treatment. There was no indication of n-3 PUFA (the most labile FA) loss as a result of thermal degradation. This thermal stability even with a drastic cooking procedure such as grilling has been reported previously( Reference Costa, Afonso and Cardoso 8 , Reference Costa, Afonso and Bandarra 13 , Reference Weber, Bochi and Ribeiro 28 ). Hence, it can be stated that potentially deleterious reactions such as oxidation were not too important( Reference Little, Armstrong and Bergan 29 ).
In addition, for instance, a meal of 160 g of grilled seabream muscle may provide 1701 mg of EPA+DHA, which means that a daily meal of grilled seabream warrants more than three times the recommended daily intake of EPA+DHA (500 mg/d) according to the American Heart Association( Reference Kris-Etherton, Harris and Appel 30 ).
The amount of linoleic acid and MUFA in farmed seabream is higher compared with wild seabream( Reference Alasalvar, Taylor and Zubcov 31 ); however, in the case of MUFA, values are lower compared with other reported farmed gilthead seabreams( Reference Wassef, Saleh and El-Hady 27 ). It should be stressed that, given the fact that high FA levels before digestion do not ensure a high intestinal absorption of these lipids, a bioaccessibility study is warranted (see below).
A deeper analysis is rendered possible with the knowledge of the relative FA profile of each main lipid class in raw and grilled seabream before digestion. This means a study of the FA composition of TAG, NEFA and PL (Table 2). In general, for both raw and grilled seabream, the FA composition of the PL was different from that of TAG and NEFA (Table 3). Indeed, the PL fraction was richer in PUFA, particularly n-3 PUFA, and poorer in MUFA. The n-3:n-6 ratio increased from 2·8–3·0 in TAG and NEFA to 4·0–4·3 in PL. In addition, palmitic acid and DHA were more concentrated and myristic (14 : 0), palmitoleic, oleic and linoleic acids were less concentrated in PL. In some cases, such as palmitic, palmitoleic, oleic and linoleic acids, the NEFA fraction occupied an intermediate position with no difference with respect to both TAG and PL.
These results agree with the literature on FA composition of farmed fish lipid classes( Reference Yildiz, Sener and Timur 32 – Reference Sargent, Tocher and Bell 36 ), as such previous studies have also reported higher palmitic acid, DHA and n-3 PUFA contents and lower myristic, palmitoleic, oleic and linoleic acid contents in PL. However, contrary to some reports( Reference Ibeas, Cejas and Fores 33 , Reference Ruiz-Lopez, Stubhaug and Ipharraguerre 35 ), there was no higher EPA content in PL than in other classes. This may be explained by the utilisation of EPA as a substrate for eicosanoid biosynthesis in marine fish species( Reference Izquierdo 37 , Reference Bandarra, Rema and Batista 38 ), thereby reducing its structural role in PL. TAG FA composition largely reflects the composition of the diet, which is typically richer in MUFA and linoleic acid because of the high portion of vegetable meal and vegetable oil used in the making of farmed fish feeds( Reference Ruiz-Lopez, Stubhaug and Ipharraguerre 35 ). This is a consequence of the role of TAG as storage fat in fish. On the other hand, PL are structural lipids in the membranes of fish cells and they require specific, bound FA in order to function properly. For example, PL rich in DHA and other n-3 PUFA provide sufficient plasticity to cope with pressure and temperature changes in the water environment( Reference Sargent, Tocher and Bell 36 ).
Finally, it should be remarked that there were no important differences between the FA profiles of the lipid classes of raw and grilled seabream muscles. Hence, grilling did not cause any fundamental shift in the distribution of the FA. As this culinary treatment does not involve any major fat addition( Reference Costa, Afonso and Bandarra 13 ), as with frying, such absence of differences can be interpreted as further evidence of lipid thermal stability.
Bioaccessibility
Protein and lipid fractions
The level of protein hydrolysis during in vitro digestion is a measure of the efficiency of the used method. It was found that after in vitro digestion of seabream, protein recovery (%) in the bioaccessible fraction was 98·5 % for raw and was slightly lower, 94·6 %, for grilled fish. Therefore, it can be claimed that almost all fish proteins were hydrolysed in raw and grilled seabream. The lower protein bioaccessibility in grilled salmon could be due to the harsh thermal treatment( Reference Dadorama 39 ). Indeed, heating leads to protein denaturation and digestibility reduction owing to the generation of covalent bonds between polypeptide chains( Reference Dadorama 39 ).
With regard to digestion of the lipid fraction (Table 4), the bioaccessible percentages, although high, were lower than that for protein, 80 (sd 5) % for raw and 74 (sd 8) % for grilled seabream, respectively. Nonetheless, lipid digestion was quite thorough, given the disappearance of the TAG band in TLC and the large increase in the NEFA portion (Table 2). PL were also digested, but some remained non-hydrolysed. Moreover, the reduction in lipid bioaccessibility with grilling was statistically significant (P<0·05) (Table 4). This seems to indicate that the protein aggregates formed as a result of cross-linking reactions induced by grilling( Reference Dadorama 39 ) were effective in trapping a significant portion of the lipids, thus reducing lipid bioaccessibility. A similar bioaccessibility reduction with grilling has been observed for farmed salmon( Reference Costa, Afonso and Cardoso 8 ).
Table 4 Lipid and fatty acid bioaccessibility (%) in raw and grilled gilthead seabream (Averages and standard deviations)
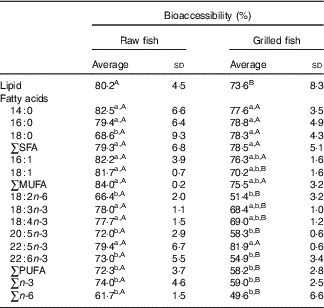
a,b For each culinary treatment, different unlike lowercase superscript letters within a column correspond to statistical differences (P<0·05) between fatty acids. A,B For lipid content and each fatty acid, different unlike uppercase superscript letters within a row correspond to statistical differences (P<0·05).
Fatty acid composition
The bioaccessibility levels for each FA in raw and grilled seabream muscles are presented in Table 4. Bioaccessibility was calculated for the most abundant FA.
FA bioaccessibility varied widely depending on the particular FA and the culinary treatment, ranging from 82·5 (sd 6·6) % for myristic acid in raw seabream to 51·4 (sd 3·2) % for linoleic acid in grilled seabream. A more systematic review of the results showed that SFA and MUFA were more bioaccessible (76–84 %) than PUFA (58–72 %) in raw and grilled seabream. This was also found in a previous study with salmon when grilled( Reference Costa, Afonso and Cardoso 8 ). The differences between bioaccessible n-3 PUFA (59–74 %) and n-6 PUFA (50–62 %) were not statistically significant. Within each main FA group, there were no major trends associated with FA chain length, with the exception of a reduction in FA bioaccessibility with stearic acid (18 : 0), a lengthier SFA, in raw fish. This effect of FA chain length in the SFA group is different from that observed for salmon( Reference Costa, Afonso and Cardoso 8 ), but is supported by other studies (although related to different food matrices), which indicate more efficient absorption into the bloodstream of SCFA and medium-chain FA( Reference Lien 40 ).
In some isolated cases, a reduction in bioaccessibility with higher levels of unsaturation was observed. For example, the pair oleic–linoleic acids, both with 18 carbons, 70–82 v. 51–66 %, supported this observation. EPA and DHA bioaccessibility (55–73 %) was clearly below that of all other main SFA and MUFA (76–83 %), with the exception of stearic acid in raw fish and oleic acid in grilled fish. Concerning this effect of the unsaturation level, it is possible that lipases are less able to hydrolyse more unsaturated FA, thereby contributing to a reduction in lipid bioaccessibility. This explanation is based on a recent study reporting that porcine pancreatic lipase (PPL) preferentially hydrolyses FA whose first double bond from the ester linkage is farther from the ester moiety( Reference Akanbi, Sinclair and Barrow 41 ). Precisely, less unsaturated FA have in average a double bond not so close to the ester linkage. According to this hypothesis, less hydrolysis for highly unsaturated FA would entail a higher portion of these in the form of TAG, DAG and MAG, which are larger molecules and may be retained in the non-digested part.
The effect of culinary treatment on fatty acid bioaccessibility
A comparison between the bioaccessibility of FA in raw and grilled seabream muscles is given in Table 4.
The reduction in lipid bioaccessibility (see above) as a result of grilling is also replicated by several specific FA. This variation was mainly detected for total PUFA, n-3 PUFA and n-6 PUFA. In particular, the bioaccessibility of oleic, linoleic, α-linolenic (18 : 3n-3), EPA and DHA was affected by the cooking method. On the other hand, palmitoleic acid, DPA (22 : 5n-3) as well as none of the SFA displayed a significant variation in bioaccessibility. It can be hypothesised that the protein aggregates formed as a result of cross-linking reactions induced by grilling( Reference Dadorama 39 ) were more effective in withholding PUFA than SFA. The higher polarity associated with double bonds may make the PUFA more prone to get attached to the protein aggregates and be partially removed from the bioaccessible fraction. However, these bioaccessibility contrasts between different FA were not observed in a previous study with salmon( Reference Costa, Afonso and Cardoso 8 ). Therefore, given the novelty of these bioaccessibility studies with lipids, further experimental studies are required.
The effect of lipid class on fatty acid bioaccessibility
For a better understanding of the bioaccessibility results, it is important to perform a more detailed analysis of the FA profiles and take into account their variation between different lipid classes in the bioaccessible fractions of raw and grilled seabream (Table 5).
Table 5 Fatty acid profile (%) of the main lipid classes in the bioaccessible fraction from raw and grilled gilthead seabream (Averages and standard deviations)

MAG, monoacylglycerol; PL, phospholipids.
a,b,c For each culinary treatment, different unlike lowercase superscript letters within a row correspond to statistical differences (P<0·05). A,B For each lipid class, different unlike uppercase superscript letters within a row correspond to statistical differences (P<0·05).
* NEFA and PL values are statistically different (P<0·05) from the initial NEFA and PL (raw and grilled) shown in Table 3.
First, it must be mentioned that the in vitro digestion caused a thorough alteration in lipid class distribution (Table 2). The NEFA were the main lipid class in the bioaccessible part of both raw (86·2 % of total fat) and grilled seabream (84·3 % of total fat). This equates to a deep change with respect to fat class distribution in seabream before digestion. Besides, TAG were not detectable, and a scarce amount of MAG was detected after digestion, 1–2 %. PL were partially hydrolysed.
Comparable results have been delivered by other studies using in vitro digestive methodologies( Reference Costa, Afonso and Cardoso 8 , Reference Gervais, Gagnon and Kheadr 42 ). Nonetheless, other studies have reported only partial TAG hydrolysis( Reference Martin, Nieto-Fuentes and Señoráns 43 ). These discrepancies may be the consequence of technical details such as the relation between fat substrate amount and lipase quantity. Contrary to previous studies( Reference Costa, Afonso and Cardoso 8 ), PL hydrolysis was achieved; however, it was not complete. The absence of total PL hydrolysis warrants further study, as no important deleterious effect on the release of NEFA from the PL in comparison with TAG has been observed( Reference Michalski, Genot and Gayet 44 ).
These results show differences between the FA profile of NEFA, MAG and PL in the bioaccessible fraction of both raw and grilled seabream. Typically, the MAG had a FA composition more similar to that of PL, whereas NEFA were different from these two classes. That is, although the total SFA in bioaccessible raw fish and n-3 PUFA in bioaccessible grilled fish were higher in the MAG and PL classes, total MUFA and n-6 PUFA were lower in these lipid classes. In particular, differences were found for oleic and linoleic acids (lower in MAG and PL) and DHA (higher in MAG and PL). Myristic and palmitic acids were particularly high in the MAG class. Conversely, stearic acid was lower in this lipid class. These results are partly explained by the differences between TAG and PL before digestion. The PL before digestion were already richer in n-3 PUFA (particularly, DHA) and poorer in MUFA (oleic acid and others) and linoleic acid. On the other hand, there were differences between the FA profiles of PL before and after digestion (Tables 3 and 5). For instance, the EPA content in the PL of raw seabream muscles was reduced from 5·6 (sd 0·1) to 3·7 (sd 0·7) %. In addition, when considering the bioaccessible fractions of each lipid class, there were a few differences between the FA profiles of raw and grilled seabream. This reinforces the conclusion that, although grilling is a drastic thermal treatment, it did not cause major damage to the chemical structure of lipid molecules.
The FA profile of MAG may be due to the selectivity of lipases during digestion of TAG. This selectivity may lead to the formation of some NEFA and the relative concentration of certain FA, such as palmitic acid and DHA, in the MAG. Moreover, lipases may operate in a selective way owing to either chemical affinity or sensitivity to the position of the FA chain in the TAG. In particular, the precise nature of PPL selectivity under in vitro digestion conditions is debatable. On the one hand, although n-3 PUFA such as DHA are very frequently bound at the second position (sn-2) of TAG molecules, two other mid- or SCFA are in the lateral (1- and 3-) positions (sn-1/3)( Reference Schuchardt and Hahn 45 ). This makes the rupture of the ester bond of a long-chain FA by PPL harder to achieve( Reference Schuchardt and Hahn 45 ). Conversely, FA in the sn-1/3 position are more easily hydrolysed, leading to the formation of 2-MAG by this enzyme. This hypothesis would be compatible with a large increase in DHA in the MAG (especially 2-MAG) class, as observed in the current study.
On the other hand, if, instead of regioselectivity, the FA chemical structure (namely, the number of double bonds) was decisive for PPL action( Reference Akanbi, Sinclair and Barrow 41 ), this would yield DAG (and may be TAG) along with MAG (1-MAG/3-MAG besides 2-MAG) enriched in DHA and other highly unsaturated FA. The results obtained in the current study do not allow a separate analysis between 1-MAG/3-MAG and 2-MAG. However, the evidence supporting the regioselectivity hypothesis may be highlighted by the enrichment in palmitic acid of the MAG class. Palmitic acid is neither very long nor unsaturated, and thus any structural selectivity against DHA hydrolysis would not apply to palmitic acid. Although stearic acid is longer than palmitic acid, it is less concentrated in the MAG class, thus further weakening the existence of a causality link between structure and hydrolysis by the lipase. Of course, a positional selectivity of PPL presupposes that besides DHA palmitic acid is more frequently bound at the second-position (sn-2) of TAG in fish. Precisely, it has been reported that most fish species have a higher proportion of palmitic acid in position sn-2( Reference Brockerhoff, Hoyle and Hwang 46 ). It is also very interesting to note that, according to this study, although myristic acid is most often concentrated in sn-2, stearic and oleic acids are most often found at sn-1/3. This fits perfectly with the current study’s results. Therefore, in vitro lipid digestion seems to confirm a predominant regioselective action of PPL.
The FA profiles of the lipid classes of the bioaccessible fractions attained from raw and grilled seabream may help explain the bioaccessibility differences between the FA (Table 4). However, although the high frequency of DHA in MAG and PL (PL were not as extensively hydrolysed as the TAG class) may be related to a lower DHA bioaccessibility – provided that PL and MAG have less affinity for the bioaccessible fraction – the low percentage of linoleic acid in MAG and PL did not correlate with a high bioaccessibility. Therefore, bioaccessibility differences between FA may be caused by the chemical interactions of their multiple forms (for instance, oleic acid, monoolein, glyceryl dioleate, glyceryl trioleate, etc.) to the aggregated protein in the non-bioaccessible fraction v. the components in the bioaccessible fraction. The higher polarity of PUFA would be decisive for a lower bioaccessibility of these FA as observed in this study.
Conclusions
In raw and grilled seabream muscles, PUFA were the most abundant FA group, followed by MUFA and SFA. The relative FA profile varied across lipid classes, being more dissimilar between TAG and PL. The latter were richer in DHA. A daily meal of grilled seabream ensures more than three times the recommended daily intake of EPA+DHA (500 mg/d).
Regarding bioaccessibility, long-chain SFA and PUFA seemed to be less bioaccessible than short-chain SFA as well as SFA and MUFA. Moreover, grilling reduced the relative portion of bioaccessible protein, fat and many FA, with the highest reductions found in PUFA such as DHA. Lipid class may have also played a role in the bioaccessibility process. However, other chemical affinity phenomena may be more influential.
Finally, it was observed that the FA composition of the MAG class correlated quite well with the position of the FA in the TAG molecule, thus providing evidence for a regioselective action of the lipase under the in vitro digestive conditions. At least for DHA, its sn-2 position at TAG reduced its hydrolysis degree and may have contributed for its bioaccessibility result.
Acknowledgements
This work was supported by the project ‘GOODFISH’, ref. no. PTDC/SAU-ESA/103825/2008, and the individual Post Doctoral Grants for the authors C. A., ref no. SFRH/BPD/64951/2009, and C. C., ref. no. SFRH/BPD/102689/2014, all of ‘Fundação para a Ciência e a Tecnologia’ (FCT).
S. C. carried out lipid analysis and data treatment; C. A. oversaw the bioaccessibility experimental; C. C. performed the calculations and statistical analysis and wrote the manuscript; R. O. and F. A. carried out the bioaccessibility experimental part together; M. L. N. devised the experimental design; and N. M. B. oversaw the performance of the trials and was involved in writing the manuscript.
The authors declare that there are no conflicts of interest.








