Breast-feeding is the ‘gold standard’ in infant nutrition. Therefore, the composition of current infant formulas is largely determined by the composition of human breast milk. Nevertheless, major differences exist between breast-fed and formula-fed infants. For example, formula-fed infants have a greater tendency to develop constipation, early wheezing and they also experience more infections in their first year of life(Reference Quinlan, Lockton and Irwin1–Reference Wright, Bauer and Naylor3). The composition of the intestinal microbiota differs between formula-fed and breast-fed infants. Breast-feeding stimulates the development of a microbiota dominated by bifidobacteria and lactobacilli, for as much as 90 % of the total gut flora. In contrast, the flora of formula-fed infants is more diverse, containing Bacteroides, bifidobacteria, staphylococci, Escherichia coli and Clostridia(Reference Yoshioka, Iseki and Fujita4–Reference Harmsen, Wildeboer-Veloo and Raangs6).
The gut flora appears to modulate the health and wellbeing of the host(Reference Guarner and Malagelada7) and it has been suggested that the observed differences in microbiota contribute to the lower incidence of infections, allergies and gastrointestinal disturbances in breast-fed infants compared with formula-fed infants(Reference Koletzko, Aggett and Bindels8, Reference Hanson and Telemo9). If so, it seems rational to adapt infant formulas to promote the establishment of an intestinal microbiota resembling that of breast-fed infants. The addition of prebiotics (non-digestible food ingredients) to infant formula has been shown to be an efficient way to increase the number of beneficial bacteria in the intestine(Reference Macfarlane, Macfarlane and Cummings10). Prebiotics are usually well tolerated and regarded as safe. Another approach to improve intestinal microbiota is to add probiotics to infant formula. The addition of 106–107 colony-forming units (CFU) of lactobacilli and/or bifidobacteria per g of infant milk powder can result in colonisation of the gastrointestinal tract(Reference Langhendries, Detry and Van Hees11, Reference Vendt, Grunberg and Tuure12). Probiotics of bacterial origin are also generally considered safe, but systemic infections with L. rhamnosus and Bacillus have occurred (as summarised by Aggett et al. (Reference Aggett, Agostini and Goulet13)). The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition recommends that infant formulas with added bacteria regarded as probiotics should be marketed only if a full evaluation of benefits and safety has been performed following the general principles as defined by Aggett et al. (Reference Aggett, Agostini and Goulet13), Koletzko et al. (Reference Koletzko, Ashwell and Beck14) and Agostoni et al. (Reference Agostoni, Axelsson and Braegger15).
The purpose of the present study was to assess the safety and tolerance of the addition of Bifidobacterium animalis ssp. lactis (also known as Bifidobacterium Bb-12) as well as Lactobacillus paracasei ssp. paracasei (also known as L. casei CRL-431) to a prebiotic-containing infant formula in a group of healthy, term infants, receiving the formula from birth onwards until the age of 6 months, by identifying any adverse effects, and by examining effects on growth, stooling habits and clinical characteristics such as infections. Bifidobacterium Bb-12 has already been studied extensively in infants. The addition of these species to regular infant feeding has been found to result in normal infant growth with an increase of the number of faecal bifidobacteria(Reference Langhendries, Detry and Van Hees11, Reference Saavedra, bi-Hanna and Moore16–Reference Mohan, Koebnick and Schildt18). L. casei CRL-431 has shown positive effects in the treatment of diarrhoea in children(Reference Gaon, Garcia and Winter19). So far, no safety studies in infants have been performed with L. casei CRL-431.
Subjects and methods
Subjects
Pregnant mothers, who intended to bottle-feed their infant from birth onwards, were recruited from five antenatal clinics in the central part of the Netherlands. Mothers who stopped breast-feeding within the first week after birth were invited to participate in the study as well. All infants had to be born after ≥ 37 weeks of gestation and had to be aged < 7 d at the time of enrolment. Exclusion criteria were the use of antibiotics in the first week, congenital illnesses or malformations that could affect normal growth, and insufficient knowledge of the Dutch language. All parents gave written informed consent. The study protocol was approved by the medical ethics committee of the hospital. This trial is registered as an International Standard Randomized Clinical Trial (no. ISRCTN 78225533).
Trial design
Infants were randomly allocated using a computerised random-number generator for concealment to either the experimental formula with probiotics or the control formula for the first trimester. Parents of the first eighty infants who completed the first part of the study were asked to continue the use of the study formula for another 3 months. Parents received the assigned infant formula with written instructions for its preparation and were advised to feed infants ad libitum during the study period. Solid foods were introduced at the age of 4 months. Parents were provided with a diary and asked to record crying and sleeping hours, and stool characteristics: frequency of stool passage and consistency (on a four-point scale of 1 = hard to 4 = watery and loose) for three consecutive days at the end of each month(Reference Tjon, Ten and Wolters20). Parents also recorded the infant's use of antibiotics, visits to their general practitioner, and periods with signs of upper respiratory tract infections and gastrointestinal infections. Furthermore, they were asked to record adverse effects (vomiting, diarrhoea, constipation, colic and rash or eczema). In case of serious adverse events, parents were instructed to contact one of the paediatricians (A. M. V. or A. R.). Anthropometric measurements (length, weight and head circumference) were taken each month at healthy baby clinics. Visits at the hospital took place at 3 months for all infants who completed the first 3 months of the study and at 6 months for those infants who participated also during their second trimester. Furthermore, faecal samples were collected at 1, 2, 3 and 6 months of age for analysis of the faecal flora. The microbiological results will be published separately.
Study formulas
The experimental and control formulas were standard milk-based powder products (Friso 1; commercially available) that when prepared in accordance with the instructions contained (per 100 ml): 284 kJ, 1·4 g protein, 1·5 g fat, 7·3 g carbohydrates, 0·24 g prebiotic galacto-oligosaccharides, minerals, vitamins, nucleotides, choline, taurine, l-carnitine and inositol. In addition, the experimental formula contained 1 × 107 CFU B. animalis ssp. lactis/g (also known as Bifidobacterium Bb-12), deposited under American Type Culture Collection (ATCC) number 27 536 and 1 × 107 CFU L. paracasei ssp. paracasei/g (L. casei CRL-431), deposited under ATCC number 55 544. Both formulas had similar taste, smell and colour and were supplied by Friesland Foods, Leeuwarden, the Netherlands. Products were manufactured according to current good manufacturing practices and coded at the manufacturing site. During storage of the product at ambient temperature, the stability of the probiotics in the product was checked monthly by selective plate counting and over a period of 2 years the CFU/g remained stable in the product.
Statistical analysis
Primary outcomes were differences in growth parameters at 3 months of age. Using data from the Social Medical Survey of Children attending Child Health Clinics (SMOCC)(Reference Herngreen, van Buuren and van Wieringen21), we estimated the weight gain between birth and the age of 3 months equal to 2578 (sd 890) g. We defined the equivalence margin equal to 500 g, and calculated that fifty-five individuals per group were needed for a statistical test using α = 0·05 and a power = 0·80. Taking a drop-out rate of 20 % into account, 132 infants had to be enrolled. Secondary outcomes were differences in growth parameters at the age of 6 months, and differences in stool characteristics (consistency and frequency), crying (h/d), the number of upper respiratory tract infections and gastrointestinal tract infections as diagnosed by the parents, the number of antibiotics and visits to the general practitioner.
Diaries and growth charts were analysed by A. M. V., A. R. and S. B. who were all blinded as to the treatment arm. Anthropometric data were expressed as sd scores according to age and sex, with respect to the Dutch references(Reference Fredriks, van Buuren and Burgmeijer22). Anthropometric data were checked by plotting the sd score by age of each child. Statistical analyses were performed according to the intention-to-treat principle. Differences between the two therapy groups were analysed by t tests, ANOVA, χ2 test and Fisher exact tests where appropriate. SPSS 14.0 (SPSS, Inc., Chicago, IL, USA) and S-PLUS® 8.0 (Insightful Corp., TIBCO Software Inc., Palo Alto, CA, USA) were used for data handling, graphing and statistical analysis.
Results
Between November 2004 and January 2007, 159 mothers agreed to participate in the study. A flowchart showing the enrolment and status of the infants is presented in Fig. 1. After birth, twenty-six infants were excluded from the study due to practical issues (n 5), prematurity (n 4), use of antibiotics (n 3), feeding problems in the first 3 d (n 3), refusal of randomisation (n 2), fear of negative effects (n 2) and unknown reasons (n 7). A total of sixty-nine infants were allocated to the probiotics group and sixty-four to the control group. In the experimental group two infants were lost to follow-up and another fourteen infants dropped out of the study within the first 3 months. In the control group, five infants were lost to follow-up and twelve infants dropped out. Reasons for dropping out were similar in both groups and included, in the experimental and control groups, respectively, colic (6 v. 4), regurgitation (1 v. 3), constipation (3 v. 4) and practical issues (4 v. 1). Thus in total 126 infants (sixty-seven in the probiotics group and fifty-nine in the control group) were considered for the intention-to-treat analysis at 3 months. Since most parents did not fill in the diaries any more after dropping out, only growth parameters could be analysed in the intention-to-treat group. The population for the per-protocol analysis consisted of fifty-three and forty-seven infants at the age of 3 months, and forty-one and thirty-eight infants at the age of 6 months, for the probiotics and control groups, respectively. Upon study entry no differences existed in anthropometric data. However, fewer infants were born by Caesarean section in the probiotics group (Table 1).

Fig. 1 Trial profile. ITT, intention to treat; PP, per protocol.
Table 1 Baseline characteristics
(Mean values and standard deviations)
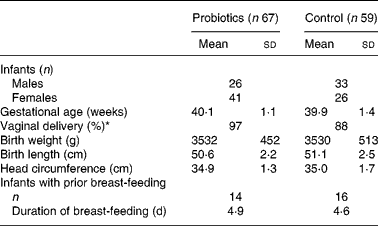
* Fewer infants were born by Caesarean section in the probiotics group (P < 0·05).
Growth
Table 2 presents the sd scores for weight, length and head circumference at birth and the age of 3 months for the intention-to-treat group, and at the age of 6 months for the per-protocol group. Normal growth occurred in all infants and no statistical differences were detected in sd change scores between the probiotics group and the placebo group for the three growth parameters during the first 3 and 6 months. The probiotics group gained 2507 g weight and 10·3 cm length in the first 3 months v. 2661 g and 10·6 cm in the placebo group (P = 0·64 for weight and P = 0·85 for length). After 6 months the absolute weight gain was 4152 g in the probiotics group v. 4282 g in the placebo group (P = 0·60) and gain in length was 17·7 v. 17·3 cm (P = 0·30). Similar results were obtained when growth increments were analysed for each month separately.
Table 2 Growth data expressed as standard deviation score scale*
(Mean values and standard deviations)
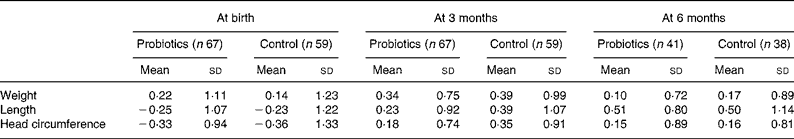
* There were no statistically significant differences detected between the probiotics and control groups.
Stool characteristics
Infants in the experimental group had a higher stool frequency during the first 3 months than those in the control group (1·52 v. 1·29 times per d; P = 0·04; Table 3). These differences became less pronounced during the second trimester (1·60 v. 1·40 times per d; P = 0·13). In the first 3 months there was a higher stool consistency-score (2·57 v. 2·36; P = 0·05) in the probiotics group, reflecting softer stools. This difference in stool consistency score was not present any more during month 4 to month 6 (2·40 v. 2·27; P = 0·36).
Table 3 Stool characteristics during the first and second trimester
(Mean values and standard deviations)
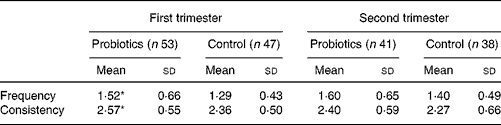
* Mean value was significantly different from that of the control group in the first trimester (P < 0·05).
Clinical outcomes
Table 4 shows the variables of general health in the group of infants who completed the study during 6 months. During the intervention no differences were found between the groups regarding crying and sleeping hours, nor in other parameters such as the number of gastrointestinal or upper respiratory tract infections, the amount of antibiotics and visits to the general practitioner. Data analyses on these clinical variables were also carried out for the first 3 months of life and for each month separately; again, results were similar in both groups (data not shown).
Table 4 Parameters of general health during the first and second trimester*
(Mean values and standard deviations)
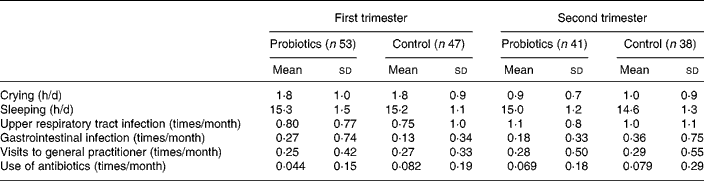
* There were no statistically significant differences detected between the probiotics and control groups.
Adverse events
No serious adverse events were reported that could be related to the study formula. Parents were asked if they had noticed any symptoms that could have been caused by the study feed (Table 5). Fewer infants in the probiotics group had developed a rash in the first 3 months (5 v. 12; P < 0·05). No differences were seen in other adverse effects between the two groups in both the first and second trimester.
Table 5 Number of infants with adverse effects as reported by the parents
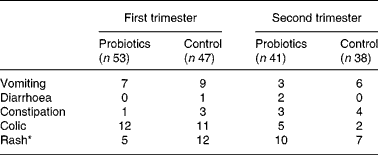
* Fewer infants experienced a rash in the probiotics group compared with in the control group in the first trimester (P < 0·05).
Discussion
The present study clearly shows that the use of a starter formula containing prebiotics and supplemented with L. paracasei ssp. paracasei and B. animalis ssp. lactis in early infancy is safe and has no adverse effect on growth and infant behaviour. A higher stool frequency and softer stools were found in those infants receiving the formula containing the probiotic strains compared with infants receiving a standard formula without these probiotics. No significant changes in sd scores for weight, length and head circumference were observed between the study and the control group. All infants had a normal linear and ponderal growth. This is in agreement with similar safety studies with probiotics and synbiotics, in which no negative effect on infant growth was reported(Reference Saavedra, bi-Hanna and Moore16, Reference Weizman and Alsheikh23–Reference Chouraqui, Grathwohl and Labaune25). These other safety studies were done with different (combinations of) probiotics. Therefore, the results of these studies cannot simply be extrapolated to other probiotic strains, since different probiotic strains have different metabolic activities.
Drop-out rates, reasons for dropping out and the incidence of adverse events were similar in both the probiotics and the control group, suggesting that the probiotic feeding was well tolerated. We did not find any difference in the incidence of colic or the number of crying hours. This is in contrast to a similar safety study in infants of 3–24 months, using B. animalis ssp. lactis and Streptococcus thermophilus, in which a lower incidence of colic and irritability in the probiotics group was reported(Reference Saavedra, bi-Hanna and Moore16). The lower age of the infants in the present study and the fact that we used L. paracasei ssp. paracasei instead of S. thermophilus may have contributed to this discrepancy. We did find a lower incidence of rash as reported by the parents in the probiotics group during the first trimester. Since the first medical examination during the study was performed at the age of 3 months, when most of the rashes had disappeared already and were thus self-limiting, we cannot discriminate between the different causes of a rash (viral, allergic, or constitutional) and can only speculate on the reason for this difference. For example, the lower incidence of a rash could reflect an influence of the probiotic strains on the development of atopic eczema, since several studies have reported a reducing effect of probiotic supplementation on the incidence of eczema in atopic infants(Reference Prescott and Bjorksten26). On the other hand, recently Hol et al. have showed that supplementation of infant formula with similar probiotics did not result in a lower incidence of atopic eczema in a group of infants with cows' milks allergy(Reference Hol, van Leer and Elink Schuurman27). For these reasons, and because there was no reduction of rash at 6 months, it seems unlikely that the lower incidence of rash at 3 months in the probiotics group in the present study reflects a modulation of the immune system.
Supplementation with L. paracasei ssp. paracasei and B. animalis ssp. lactis was associated with an increase in defecation frequency and softer stools during the first trimester. Several other studies in infants have documented similar effects of probiotics or synbiotics on stool frequency and/or consistency(Reference Vendt, Grunberg and Tuure12, Reference Puccio, Cajozzo and Meli24, Reference Chouraqui, Grathwohl and Labaune25). Furthermore, advantageous effects of probiotics on stool consistency and frequency have been reported in constipated children(Reference Bekkali, Bongers and Van den Berg28, Reference Bu, Chang and Ni29). Constipation is a frequent symptom in bottle-fed infants with prevalences of up to 17 %(Reference Quinlan, Lockton and Irwin1, Reference Infante Pina, Badia Llach and Ariño-Armengol30). It would therefore be interesting to explore the significance of our findings in a larger study in infants, adequately powered to examine possible differences in incidence of constipation. Such a study may also help to answer the question whether the presence of prebiotics such as galacto-oligosaccharides are a prerequisite to induce the effect of probiotics on stool consistency and frequency.
Another possibility is to address the effects of the combination of these two probiotic strains on infants who already have developed constipation. The difference in stool characteristics between the two study groups disappeared in the second trimester. We hypothesise that this is due to the fact that the infants started with solid foods in the 4th month, thereby influencing the gut flora significantly. Additional studies, examining the flora of probiotic-fed infants, may help to shed light on this question and also help to unravel the still unknown mechanisms by which probiotics can increase faecal moisture and enhance gastrointestinal motility.
It has been reported that B. animalis ssp. lactis reduces the incidence of infectious diarrhoea and the use of antibiotics in infants(Reference Saavedra, bi-Hanna and Moore16, Reference Chouraqui, Van Egroo and Fichot31, Reference Weizman, Asli and Alsheikh32). Furthermore, L. casei CRL-431 has shown positive effects in the treatment of diarrhoea in children(Reference Gaon, Garcia and Winter19). We therefore asked parents to report periods with signs of a gastrointestinal or respiratory infection, use of antibiotics and visits to the general practitioner. In contrast to the above-mentioned studies, we did not find any difference in these parameters between infants with and without probiotics in their formula feeding. The apparent lack of preventive effect by the combination of these two probiotics could be related to the fact that infants in the present trial were much younger than those in the other studies and visited a daycare centre less often, probably resulting in lower risks of developing infections. A longer follow-up study will be necessary to reveal any protective effects of this combination of probiotics against infectious diseases.
The present study is not without limitations. First, our drop-out rate was higher than expected (thirty-three out of 133), resulting in a slightly lower number of children that completed the first treatment period of 3 months than had been calculated in our power analysis. A drop-out rate of 25 % is not uncommon in these kind of studies(Reference Saavedra, bi-Hanna and Moore16, Reference Puccio, Cajozzo and Meli24, Reference Chouraqui, Grathwohl and Labaune25). It is known that parents often switch formulas in the first 6 months of life (up to 47 %) because of concerns regarding common infantile symptoms perceived by parents to be related to formula intolerance(Reference Nevo, Rubin and Tamir33). The decision to switch formula is often made without consulting a health professional. This also occurred in the present study: most of the parents who decided to switch formula did so without notifying the investigators. Since the drop-out rates and the reasons for dropping out in the present study were similar in both treatment groups and because almost all drop-outs occurred in the first 6 weeks, we assume that the higher than expected number of drop-outs was unrelated to the probiotics. Although the study formula was delivered at home at parents' request and parents were repeatedly instructed to use only the study formula, we cannot rule out the possibility that compliance with the protocol was suboptimal, since we did not monitor the intake of the study formula. However, we could confirm the intake of probiotics by faecal analysis, which identified the specific bacterial strains mainly in the probiotics group (F Schuren, AM Vlieger, A Nauta and R te Biesebeke, unpublished results). Third, we studied the number of infections only by parental observation instead of confirming these infections by examining infants and taking cultures of stools and nose swabs. This could potentially affect the conclusion that the number of infectious periods was similar in both groups. Finally, there were more vaginal deliveries in the probiotics group. It is known that the number of bifidobacteria in the faecal flora is significantly higher after vaginal deliveries in comparison with Caesarean sections(Reference Penders, Thijs and Vink34) and this may have been of influence on the difference in stool consistency and frequency, as found in the present study. Faecal analysis of both infant groups, focusing on these potential differences in bifidobacteria, may answer the questions if this difference in mode of delivery has indeed resulted in a difference in the composition of the flora of both groups and if it was related to changes in stool pattern.
In conclusion, this safety study demonstrates that the administration of a prebiotic-containing starter formula supplemented with L. paracasei ssp. paracasei and B. animalis ssp. lactis to infants is safe, well tolerated, does not adversely affect growth and infant behaviour in the short term and can result in softer and more frequent stools.
Acknowledgements
We thank Marielle Bink and Ralph Witteveen for assistance with data collection.
The study was financed by Friesland Foods, Leeuwarden, The Netherlands.
A. M. V. participated in study design and examination of the infants, coordinated data analysis and interpretation, and was responsible for writing the report. A. R. participated in examination of the infants and data collection. S. v. B. was responsible for data analysis and interpretation. J. K. was responsible for the study design. G. R. participated in data interpretation and writing of the report. M. A. B. contributed to the study design, data interpretation and writing of the report. R. t. B. coordinated the project. All authors have seen and approved the final version.
J. K. and R. t. B. were employed by the sponsor (Friesland Foods, Leeuwarden, the Netherlands) at the time of the study. All other authors declare that they have no conflict of interest.








