Preterm infants are more susceptible to vitamin E (α-tocopherol) deficiency because they are born with low body reserves and low serum levels of this micronutrient( Reference Bell, Hansen and Brion 1 ). This deficiency may stem from limited placental transfer, low body fat, tocopherol accumulation that occurs mainly during the third trimester of pregnancy associated with an increase in body fat and higher nutritional requirements( Reference Debier 2 , Reference Dror and Allen 3 ). As a consequence, newborns are more susceptible to infections, thrombocytosis, haemolytic anaemia, retrolental fibroplasia, intraventricular haemorrhage, bronchopulmonary dysplasia and spinocerebellar ataxia( Reference Brion, Bell and Raghuveer 4 ).
Appropriate nutritional support should be provided for preterm infants to prevent the damage caused by vitamin E deficiency, and breast milk is considered the ideal food for meeting all of the newborn’s nutritional requirements( Reference Edmond and Bahl 5 ). Breast milk has been associated with many benefits such as better immunity, digestion, nutrient absorption, gastrointestinal function and neurodevelopment( Reference Lawrence and Lawrence 6 , Reference Isaacs, Morley and Lucas 7 ).
However, preterm infants require higher serum levels of the antioxidant α-tocopherol than those reached with routine oral doses of vitamin E( Reference Roberts and Knight 8 ) to eliminate free radicals and reduce the risk of lipid peroxidation and oxidative injury( Reference Thibeault 9 ).
Experts have suggested giving vitamin E supplements to preterm infants to prevent vitamin E deficiency( Reference Bell, Hansen and Brion 1 , Reference Delvin, Salle and Claris 10 ); however, many challenges are associated with vitamin and trace-element supplementation of newborns because their basic requirements, appropriate administration route and proper dosage are not well defined and vary considerably( Reference Brion, Bell and Raghuveer 11 – Reference Bell, Hansen and Brion 13 ). Moreover, breast milk vitamin E concentrations may fall during lactation( Reference Lima, Dimenstein and Ribeiro 14 ). Therefore, fortification of breast milk through maternal vitamin E supplementation may be a good way to protect newborns from the effects of vitamin E deficiency. The objective of the present study was to assess the effect of vitamin E supplementation on the α-tocopherol concentrations of colostrum, transitional milk and mature milk of women who had given birth prematurely.
Methods
Participants
The participants were women aged between 18 and 45 years seen at the Maternity Hospital Escola Januário Cicco and Pediatric Hospital Professor Heriberto Ferreira Bezerra (HOSPED), both located in the city of Natal, RN, Brazil. All the women had delivered preterm (gestational age <37 weeks). Gestational age was calculated based on the date of the last menstrual period or ultrasounds. The women joined the study between November 2013 and July 2014. Exclusion criteria were as follows: use of multivitamins during pregnancy, presence of diabetes, high blood pressure, neoplasm, gastrointestinal disease, liver disease, heart disease, infectious disease and syphilis and positive HIV status.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures were approved by the Research Ethics Committee of the Federal University of Rio Grande do Norte in compliance with Resolution 466/12 and under protocol number 461464. The clinical trial was approved by the Brazilian Registry of Clinical Trials under the number RBR-9gycqb. All the participants were informed of the study objectives and they signed an informed consent form before joining the study.
The minimum sample size was calculated by the software G*Power version·3.1.7( Reference Faul, Erdfelder and Lang 15 ) using the following parameters: an α of 5 %, power of 80 % and expected effect of 0·30. Therefore, each group should have had at least twenty individuals.
Study intervention
This randomised-controlled trial had two treatment arms: a treatment-free control group (CG) and a supplemented group (SG) that received a single oral dose of 400 IU natural vitamin E in the form of RRR-α-tocopheryl acetate. The dosage of 400 IU RRR-α-tocopherol was chosen because it has been considered safe by the Institute of Medicine( 16 ), which conducted long-term studies to investigate the toxic effect of this dosage and found no deleterious effects.
The supplement was given after the first blood and colostrum collection, within 48 h of delivery. Randomisation followed the daily delivery order: the first woman to deliver was placed in the CG, the second in the SG and so on. Initially both groups had the same number of participants, but some were lost to follow-up after hospital discharge or newborn death.
Data collection
Obstetric, newborn and maternal socio-economic data were collected from all the participants by the administration of a structured questionnaire by the researcher. The gestational age of the newborn was calculated based on the date of the last menstrual period or ultrasound. Pre-gestational nutritional status was given by the mother’s pre-gestational BMI, where maternal weight is divided by the square of maternal height (kg/m2)( 17 ).
Blood (5 ml) and colostrum (2 ml) were collected from all the participants within 48 h of delivery. The SG received the vitamin E capsule immediately after this baseline blood and breast milk collection (0-h milk). Colostrum (2 ml) was again collected from both the groups 24 h after the first collection (24-h milk), transitional milk (2 ml) was collected 7 d (7-d milk) after delivery and mature milk (2 ml) was collected 30 d after delivery (30-d milk). All the samples were collected after an overnight fast.
Blood was obtained by venepuncture and centrifuged for 10 min at 1073 g to separate 1 ml of serum. Breast milk was collected manually from the breast, which had not been suckled for at least 2 h. The first droplets were discarded to avoid fat content variations. A 500-μl aliquot of colostrum and 1 ml of transitional and mature milk were separated for vitamin E extraction. All samples were stored at −20°C until analysis.
To determine whether colostrum, transitional milk and mature milk met the vitamin E requirements of infants aged 0–6 months (4 mg/d)( 16 ), we considered that preterm infants ingest 254 ml/d of breast milk during the 1st week of life, as suggested by Bauer & Gerss( Reference Bauer and Gerss 18 ), and 552 ml/d of mature milk at 1 month of age( Reference Souza, Dolinsky and Matos 19 ).
Chemical analyses
α-Tocopherol was extracted from breast milk as recommended by Ortega et al.( Reference Ortega, López-Sobaler and Martínez 20 ), with adaptations. α-Tocopherol concentrations were determined by HPLC using a Shimadzu LC-20AT chromatograph (Shimadzu Corporation), with an injector loop of 20 μl, connected to a CBM 20A communicator (Shimadzu Corporation) and SPD-20A UV-VIS detector (Shimadzu Corporation). Chromatographic separation was achieved using a LiChroCART 250–4 reverse-phase column (Merck). α-Tocopherol was identified and quantified by comparing the area of the chromatographic peak with the respective α-tocopherol standard (Sigma®). The concentration of the standard was confirmed by the extinction coefficient specific for α-tocopherol (1 %, 1 cm=75·8 for 292 nm) in absolute ethanol (Merck®)( Reference Nierenberg and Nann 21 ). Mothers with serum α-tocopherol levels lower than 12 µmol/l (516 µg/dl) were considered vitamin E deficient( 16 ).
Statistical analysis
α-Tocopherol concentrations in the serum and breast milk of the CG and SG were compared using IBM SPSS Statistics version 21.0 for Windows (SPSS Inc.). The significance level was set at 5 % (P<0·05). The Kolmogorov–Smirnov test was used to verify sample symmetry. The Mann–Whitney and χ 2 tests were used to compare the maternal and newborn characteristics of the two groups.
The Mann–Whitney test compared the variables that were not normally distributed (vitamin E in serum, 0-h milk and 24-h milk). The t test for independent measurements compared variables with near-normal distribution (7-d and 30-d milks) and verified variance homogeneity.
Results
A total of eighty-nine women (fifty-one in the CG and thirty-eight in the SG) met the inclusion criteria. New sample losses occurred throughout the study starting on day 7. Most losses were due to infant death, no production of breast milk and dropouts. Therefore, the number of participants per collection were as follows: 7 d (n 42) and 30 d (n 33) for the CG and 7 d (n 33) and 30 d (n 22) for the SG.
Table 1 shows the general characteristics of the mothers and newborns. The CG and SG had similar characteristics.
Table 1 Socio-economic and obstetric characteristics of participating mothers and their newborns (Mean values and standard deviations; numbers and percentages)

* Mann–Whitney test.
† χ 2 Test.
The mean α-tocopherol concentration in the serum of the control mothers (1159·8 (sd 292·4) μg/dl; 27·0 (SD 6·8) μmol/l) did not differ significantly from that of the supplemented mothers (1128·3 (sd 407·2) μg/dl; 26·2 (SD 9·5) μmol/l) (P>0·05) (Fig. 1). All mothers had appropriate serum concentrations of vitamin E.
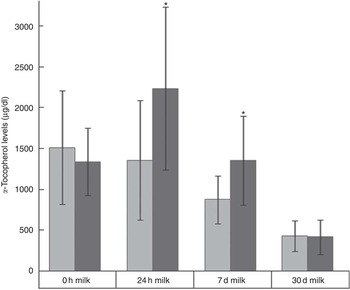
Fig. 1 Mean α-tocopherol concentrations (μg/dl) in the 0-h and 24-h colostrum, 7-d transition milk and 30-d mature milk of the control and supplemented groups. * Significant difference: P<0·05. ![]() , Control;
, Control; ![]() , supplemented. To convert α-tocopherol in μg/dl to μmol/l, multiply by 0·023256.
, supplemented. To convert α-tocopherol in μg/dl to μmol/l, multiply by 0·023256.
The CG and SG had similar α-tocopherol concentrations in the 0-h milk (P>0·05). However, the SG had higher α-tocopherol concentrations in the 24-h and 7-d milks (P<0·001). The 7-d milk of the SG had 35 % more α-tocopherol than that of the controls. The α-tocopherol concentrations in the 30-d milk of the two groups did not differ significantly (P>0·05).
The α-tocopherol concentrations in the 0-h and 24-h milk samples of the CG did not differ significantly, but the 24-h milk of the SG was 60 % higher in α-tocopherol compared with the 0-h milk (Fig. 2). The 24-h milk of the SG met 145 % of the α-tocopherol requirement of children aged 0–6 months.

Fig. 2 Infant α-tocopherol requirement and amount of α-tocopherol provided per day based on the amount of milk consumed on different occasions. ![]() , Control;
, Control; ![]() , supplemented; AI, adequate intake.
, supplemented; AI, adequate intake.
The α-tocopherol concentrations of breast milk decreased over time even in the SG. After the significant rise seen in the 24-h milk of the SG, the α-tocopherol concentration gradually decreased.
Discussion
Infant prematurity is associated with many maternal factors such as low pre-gestational weight, age extremes, history of stillbirth or many caesarians, smoking and insufficient weight gain during pregnancy, high blood pressure, genital and urinary tract infections, five or fewer prenatal visits and low educational levels( Reference Nascimento 22 , Reference Barros, Victora and Barros 23 ). The study population presented some risk factors for prematurity.
The serum α-tocopherol concentrations of the two groups were appropriate; none of the mothers were vitamin E deficient. Rodríguez et al.( Reference Rodríguez, Alonso and Sintes 24 ), Garcia et al.( Reference Garcia, Ribeiro and Araújo 25 ) and Dimenstein et al. ( Reference Dimenstein, Medeiros and Cunha 26 ) reported similar results for women who had a term birth. Weber et al.( Reference Weber, Stuetz and Bernhard 27 ) and Baydas et al.( Reference Baydas, Karatas and Gursu 28 ) studied women who had a preterm birth in Germany and Turkey, respectively, and they found higher serum α-tocopherol concentrations than those observed in the present study, which may be explained by the different dietary habits in those two countries compared with Brazil.
The vitamin E levels in the 0-h colostrum of the CG and SG were similar to those reported by other studies of women who had term births in Brazil( Reference Dimenstein, Medeiros and Cunha 26 , Reference Clemente, Ramalho and Lima 29 ), Turkey( Reference Orhon, Ulukol and Kahya 30 ) and the USA( Reference Gossage, Deyhim and Yamini 31 ) as well as women who had preterm births in Spain( Reference Quiles, Ochoa and Ramirez-Tortosa 32 ) and Brazil( Reference Grilo, Lira and Dimenstein 33 ).
Only the 24-h colostrum of the SG reached the recommended vitamin E level (adequate intake of 4 mg/d) for infants aged 0–6 months. However, this recommendation concerns term infants who consume on average 780 ml of milk/d( 16 ), which is very different from the amounts consumed by preterm infants. It is possible that the vitamin E requirement of preterm infants exceeds that of term infants; however, future studies are still needed to determine preterm infant vitamin E requirements.
In order to maintain adequate levels of vitamin E in mature breast milk, mothers should consume dietary vitamin E sources and/or vitamin E supplements( Reference Tokuşoğlu, Tansuğ and Akşit 34 ). This is especially important for those who have had preterm births, as they are a risk group for nutritional deficiencies( Reference Brion, Bell and Raghuveer 4 ).
In the present study, maternal supplementation with a megadose of natural vitamin E increased the α-tocopherol levels of the 24-h and 7-d milks, but did not affect that of the 30-d milk. A limiting factor of the study is that the initial number of participants was reduced. Although there was loss to follow-up for the analysis of α-tocopherol in mature milk, the values found were consistent with previous studies that have not administered supplements. Thus, this suggests that the effect of supplementation with one megadose was not sustained until 30 d after delivery, and it was not influenced by the sample reduction. In addition, longitudinal studies typically have decreased population over time. The positive effect of supplementation on colostrum and transition milk may be related to the elevated synthesis of fatty acids by the mammary gland in the first few days after delivery( Reference Boersma, Offringa and Muskiet 35 ), as vitamin E is related to lipid metabolism. Jaster & Wegner( Reference Jaster and Wegner 36 ) observed that lipolysis increases in adipose tissue in the last few weeks of gestation, which may be mediated by higher circulating levels of growth hormone. Growth hormone is lactogenic and essential for human postnatal growth and lipid, protein and mineral metabolism. Regarding lipid metabolism, growth hormone promotes the release of fatty acids and glycerol from adipose tissue and increases the circulation of free fatty acids and their oxidation in the liver( Reference Hurley, Black and Papas 37 ). Therefore, lactation-related hormones that affect lipid metabolism probably increase the number of mammary gland receptors that capture circulating lipids, including LDL receptors, as LDL is the main vitamin E transporter. Perinatal increase in mammary LDL receptor activity was observed by Debier & Larondelle( Reference Debier and Larondelle 38 ).
Studies have shown that the transport of vitamin E to breast milk occurs via distinct and independent mechanisms, in a controlled manner and is limited by receptors, because maternal serum α-tocopherol level is not related to colostrum α-tocopherol level( Reference Dimenstein, Medeiros and Cunha 26 , Reference de Lira, Lima and de Medeiros 39 ). Alternatively, the α-tocopherol receptors of the mammary gland may become saturated with high serum tocopherol levels because breast milk tocopherol levels do not increase proportionally.
The mechanisms by which α-tocopherol is incorporated into milk have not been entirely clarified, but α-tocopherol seems to reach the milk by LDL receptors and some through LDL captured by a process mediated by non-LDL receptors – namely, the cell surface receptors SR-B1 – which bind HDL and LDL without lipoprotein internalisation( Reference Debier 2 ). There is also the lipoprotein lipase (LPL) pathway observed in rat experiments( Reference Martínez, Barbas and Herrera 40 ). LPL activity may be high in the mammary gland and low in adipose tissue, capturing circulating α-tocopherol and transferring it to the milk( Reference Amazan, Cordero and López-Bote 41 ).
Another hypothesis is that plasma α-tocopherol is transferred to milk according to Michaelis–Menten kinetics for transmembrane active transport( Reference Mardones and Rigotti 42 ), using distinct mechanisms that transport vitamin E to the mammary gland regardless of plasma concentration( Reference de Azeredo and Trugo 43 ).
Maternal use of vitamin E supplements may also increase the body reserves of this micronutrient. Li et al.( Reference Li, Sen and Ren 44 ) found that α-tocopherol supplements have a higher impact on tissue levels of α-tocopherol than dietary α-tocopherol. Studies on maternal supplementation of vitamin E after delivery and its effect on breast milk are scarce and inconclusive; some found a correlation between supplementation and breast milk α-tocopherol concentration and some did not( Reference Lima, Dimenstein and Ribeiro 14 ). Clemente et al.( Reference Clemente, Ramalho and Lima 29 ) assessed maternal supplementation using a natural vitamin E supplement similar to that used in the present study and found similar levels of colostrum vitamin E 24 h after supplementation.
Our results provide evidence of the importance of maternal supplementation with vitamin E, especially in women with preterm births, in increasing breast milk α-tocopherol concentrations. Preterm infants have very low body fat and low reserves of fat-soluble vitamins including vitamin E( Reference Dju, Mason and Filer 45 ). Moreover, their red blood cell tocopherol concentrations are low at birth( Reference Kelly, Rodgers and Handel 46 ), making them functionally vitamin E deficient( Reference Cornblath, Gordon and Nitowsky 47 ). As a consensus on parenteral and enteral supplementation does not exist, maternal supplementation is a safe and excellent alternative for meeting the vitamin E requirements of newborns, supplying enough vitamin E to protect their bodies against oxidative stress. If supplementation is not done correctly, it may intoxicate the newborn and cause severe health damage( Reference Brion, Bell and Raghuveer 4 ).
However, a single megadose of 400 IU does not seem to be enough to increase the α-tocopherol levels of breast milk for a prolonged period of time. New studies are being developed by this research group to better assess vitamin E dosages and intervals without placing the mother–child dyad at risk of intoxication.
Acknowledgements
The authors thank the Januário Cicco Maternity School, the Postgraduate Programme in Biochemistry at Federal University of Rio Grande do Norte and all the mothers who participated in this study.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. No honorarium, grant or other form of payment was given to anyone to produce the manuscript.
R. D., K. D. d. S. R. and R. A. M. d. N. participated in the conception and design of the study; J. F. P. M., K. D. d. S. R., M. S. R. L., A. C. P. L., R. C. S. D. and A. B. d. S. participated in the acquisition, analysis and interpretation of the data; J. F. P. M. drafted the article. All the authors critically reviewed the manuscript and approved the final version submitted for publication.
There are no conflicts of interest.






