The relationship between thymus, the immune system and malnutrition
It has been a long time since scientists noticed that, in the context of the malnutrition-related immunodeficiency, the thymus undergoes a variety of alterations, comprising, among others, a severe atrophyReference Chandra1. This is so consistent that the thymus has been considered as a barometer of malnutrition Reference Prentice2. Interestingly, such thymic atrophy can also be found in a variety of infectious diseasesReference Savino3. Considering that in many countries malnutrition frequently parallels infections, these two pathological situations likely cause profound alterations in the host's immune system, likely in part as a consequence of targeting the thymus. Herein we will review the similarities concerning the changes seen in the thymus of individuals suffering from malnutrition and/or infectious diseases. Nevertheless, before compiling and discussing these data, it is worthwhile to provide a general background of the normal thymus structure and function, including the thymic microenvironmental compartment and its role in intrathymic T cell differentiation.
The thymic microenvironment and its role in T-cell differentiation
The thymus is a primary lymphoid organ, in which bone marrow-derived T-cell precursors undergo differentiation, ultimately leading to migration of positively selected thymocytes to the T cell-dependent areas of peripheral lymphoid organs. Such a process involves sequential expression of various proteins and rearrangements of the T-cell receptor (TCR) genes. Most immature thymocytes express neither the TCR complex nor the CD4 or CD8 accessory molecules; such cells are termed double-negative thymocytes, and they represent 5 % of total thymocytes. Maturation progresses with the acquisition of CD4 and CD8 markers, generating the CD4+CD8+ double-positive cells, which comprise 80 % of the whole population. In this stage, TCR genes are rearranged, and productive rearrangements yield the membrane expression of the TCR (complexed with the CD3) in low densities (TCRlow). Thymocytes that do not undergo a productive TCR gene rearrangement die by apoptosis, whereas those expressing productive TCR will interact with peptides presented by molecules of the major histocompatibility complex (MHC), expressed on microenvironmental cells. This interaction will determine the positive and negative selection events, crucial for normal thymocyte differentiation.
Negative selection results in apoptosis-mediated cell death. Positively selected thymocytes progress to the mature TCRhighCD4+CD8− or TCRhighCD4− CD8+ single positive stage, comprising 15 % of thymocytes that ultimately leave the organ to form the large majority of the peripheral T cell repertoireReference Savino and Dardenne4. Thymocyte differentiation occurs as cells migrate within the thymic lobules: TCR− CD4− CD8− and TCR+CD4+CD8+ are cortically located, whereas mature TCR+CD4+CD8− and TCR+CD4− CD8+ cells are found in the medulla. Along this journey, thymocytes interact with various components of the thymic microenvironment, a three-dimensional network formed of thymic epithelial cells (TEC), macrophages, dendritic cells, fibroblasts and extracellular matrix components. In addition to the key interaction, involving the TCR/peptide-MHC, in the context of CD8 or CD4 molecules, the thymic microenvironment influences thymocyte maturation via adhesion molecules and extracellular matrix (ECM); interactions that are relevant for thymocyte migrationReference Savino, Mendes da Cruz, Silva, Dardenne and Cotta de Almeida5, Reference Savino, Mendes-da-Cruz, Smaniotto, Silva-Monteiro and Villa-Verde6. Moreover, microenvironmental cells modulate thymocyte differentiation by soluble polypeptides, comprising: a) cytokines, such as interleukin (IL)-1, IL-3, IL-6, IL-7, IL-8 and stem cell factor; b) chemokines and c) thymic hormones, including thymulin, thymopoietin and thymosin-α1Reference Savino and Dardenne4.
Phenotypic and functional changes of thymocytes in malnutrition and acute infectious diseases
As stated above, one of the most conspicuous changes in malnutrition is thymic atrophy (Fig. 1). This phenomenon is largely due to massive thymocyte death: the main phenotypic feature of this depletion is the loss immature CD4+CD8+ cellsReference Chandra1. In addition to the increase in thymocyte death seen in thymuses from malnourished individuals, thymocyte proliferation seems to be affected, since the numbers of thymic cells labelled with proliferating cell nuclear antigen (PCNA) marker are diminished in malnourished ratsReference Mitsumori, Takegawa, Shimo, Onodera, Yasuhara and Takahashi7. Thus, the overall malnutrition-related thymocyte depletion seems to result from enhanced thymocyte death plus decreased thymocyte proliferation. It is important to highlight that the major change in the thymic lymphoid compartment is also observed in humans suffering from malnutrition: a severe thymic atrophy with cortical thymocyte depletion is a consistent finding in necropsies of malnourished subjectsReference Chandra1, Reference Lyra, Madi, Maeda and Savino8. Indeed, by means of echography, atrophy of the organ has also been observed in vivo in malnourished childrenReference Parent, Chevalier, Zalles, Sevilla, Bustos, Dhenin and Jambon9. Importantly, a study conducted in Guinea-Bissau revealed that thymus size at birth was associated with infant mortalityReference Aaby, Marx, Trautner, Rudaa, Hasselbach, Jensen and Lisse10. Nevertheless, malnutrition-associated thymic atrophy seems to be reversible if appropriate diet is provided. This concept emerges from an interesting longitudinal study carried out on severely malnourished Bolivian children that were under refeedingReference Chevalier, Sevilla, Zalles, Sejas, Belmonte, Parent and Jambon11. In this study thymus size was assessed weekly by mediastinal ultrasound scanning. Compared to controls, the malnourished group had severe involution of the thymus, a significantly higher proportion of circulating immature T lymphocytes and a lower proportion of mature T lymphocytes. After two months of diet rehabilitation, the thymic area was recovered.
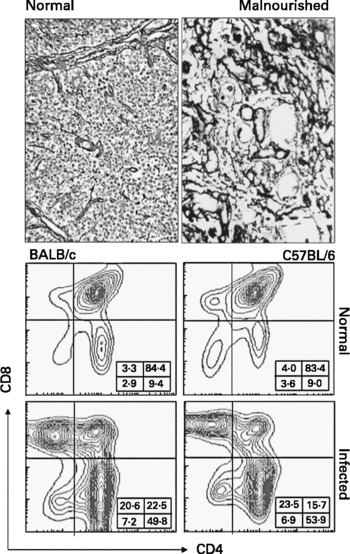
Fig. 1 Thymic atrophy in human malnutrition and experimental acute Chagas disease. Upper panel shows reticulin staining to show the increase in the extracellular matrix network seen in atrophic thymus from a malnourished child, as compared to a normal age-matched pattern. The bottom panels show the typical CD4/CD8-defined cytofluorometric profiles of thymocytes from normal or Trypanosoma cruzi-acutely infected mice. Note the large reduction in the proportions of CD4+CD8+ thymocyte subset.
Severe thymic atrophy is also a common feature in acute infections, also reflecting intense lymphocyte depletion, particularly of cortical thymocytes bearing the phenotype CD4+CD8+ (Fig. 1, Table 1). This has been shown in a variety of infections, such as AIDS, rabies, malaria, Chagas disease and schistosomiasis, among othersReference Savino3. In some cases, thymocyte loss is so severe that the cortical region of thymic lobules virtually disappears, as a consequence of the severe CD4+CD8+ thymocyte depletionReference Savino3.
Table 1 Thymic atrophy in human and experimental infectious diseases*

* Modified fromReference Savino3. ND, not determined.
Another similarity to what is seen in malnutrition, the proliferative response of thymocytes is also reduced in acutely infected individuals. We found a significant decrease in both concanavalin A- and anti-CD3-driven proliferative responses in murine Chagas disease and this outcome was paralleled by a decrease in IL-2 productionReference Leite-de-Moraes, Minoprio, Dy, Dardenne, Savino and Hontebeyrie-Joskowicz12.
Thymocyte depletion associated with specific deficiencies in vitamins and trace elements: the zinc-deficiency paradigm
In addition to protein-related malnutrition, mineral and vitamin deficiencies cause thymic atrophy, with cortical thymocyte depletionReference Kuvibidila, Dardenne, Savino and Lepault13–Reference Nodera, Yanagisawa and Wada16. Moreover, in iron-deficient mice, a decrease in mitogen-induced proliferative response of thymocytes has been demonstratedReference Kuvibidila, Dardenne, Savino and Lepault13.
Many of the effects of protein-calorie malnutrition on immune function are well recognized to result in associated alterations in mineral and/or vitamin metabolism, able to affect the immune system directlyReference Cunningham-Rundles, McNeeley and Moon17. In this regard, the abnormal incidence of various infections and the existence of lymphopaenia and lymphoid organ atrophy in malnourished children have been repeatedly demonstrated and evidence points to zinc insufficiency as being central to this type of immunodeficiencyReference Shankar and Prasad18. Zinc plays a major role in cell division, differentiation, apoptosis and gene transcription, and strongly influences the immune system affecting primarily T cellsReference Chesters, Odel, Sunde and EDS19. Studies of the effects of severe zinc deficiency in several species, including humans, report substantial thymic atrophy as well as accelerated lymphopaenia, leading to a reduction in cell and antibody-mediated responses, thus influencing the susceptibility to infectious diseasesReference Fraker, King, Garvy, Medina and Klurfeld20–Reference Fraker, King, Laakko and Vollmer22. Early observations showed that mice maintained on a zinc deficient diet develop a progressive thymic involution: after 4 weeks the thymus retains only 25 % of its original size and at 6 weeks, only a few thymocytes remain in the organReference Fernandes, Nair, Once, Tanaka, Floyd and Good23. Such changes are observed mostly in the thymic cortex, with severe loss of CD4+CD8+ thymocytes, and can be reversed by zinc supplementationReference Fraker, Depascale-Jardieu, Zwickl and Luecke24, Reference King, Osati-Ashtiani and Fraker25. Moreover, marginal zinc deficiency, in the early post-natal period, also results in substantial reduction in thymic sizeReference Beach, Gershwin and Hurley26.
The mechanism(s) of heightened apoptosis in zinc deficient mice remain(s) to be precisely determined. However, glucocorticoid hormones seem to be involved, since zinc deficiency yields a chronic stimulation of corticosterone productionReference Fraker, Osati-Ashtiani, Wagner and King27, and adrenalectomy prevents thymic atrophy secondary to zinc deficiency.
Although these studies have been performed in animal models, they raise concern about the impact of cell death in humans who are deficient in zinc due to suboptimal diets or chronic diseasesReference Fraker28. Nutritional supplementation might be considered in chronically ill patients with compromised immune defense, as in the case of AIDS patientsReference Baum, Shor-Posner and Campa29, Reference Baum, Campa and Lai30. In these subjects, zinc supplementation resulted in a significant increase in CD4+ cells and a decreased mortality. This notion can also be applied in patients with Chagas disease, since they exhibit a decrease in serum zinc concentrationsReference Burguera, Burguera, Alarcon, Canada de Zunzunegui, Carrasco, Davila and Reinosa31; the same being observed in a variety of haemopoietic organs of infected ratsReference Matousek de Abel de la Cruz, Burguera, Burguera and Anez32. Accordingly, severity of experimental Chagas disease is much higher in zinc-deficient miceReference Fraker, Caruso and Kierszenbaum33.
The hormonal control of thymocyte depletion in malnutrition and acute infections
It is now well established that the physiology of the thymus (including both lymphoid and microenvironmental compartments) is under neuroendocrine controlReference Savino and Dardenne4. In this context, it is conceivable that distinct pathological states in the organism (i.e. malnutrition and acute infectious diseases) can affect such a hormonal influence upon the thymus. Circulating levels of glucocorticoids increase in protein malnourished mice, as compared to age-matched controls. Additionally, implanted corticosterone-containing pellets, capable of generating serum glucocorticoid levels equivalent to those found in malnourished mice, are sufficient to yield thymocyte depletionReference Barone, O'Brien and Stevenson34. As discussed below, leptin also seems to be involved.
Leptin plays a complex and wide-ranging role in the regulation of physiological events that respond to the nutritional status in mammalsReference Munzberg and Myers35. Initially, leptin was described as a soluble factor that informs the hypothalamus about the energy stores in peripheral tissues, particularly about white adipose tissue. In this organ, leptin is produced and secreted in direct proportion to the total mass of adipose tissue of the organismReference Friedman36. By acting predominantly in hypothalamic neurons located in the arcuate nucleus, leptin controls neurotransmitter production and release, which leads to reduction of food intake and stimulation of energy expenditure by thermogenesisReference Munzberg and Myers35. Additionally, some humans and rodents lacking proper leptin production or to express defective leptin receptors, bear a certain degree of immunodeficiency characterized by reduced T-cell proliferative response to various mitogens, impaired production of IL-4, and inappropriate antibody production after immunizationReference Munzberg and Myers35, Reference Lord, Matarese, Howard, Baker, Bloom and Lechler37, Reference Farooqi, Matarese and Lord38. Interestingly, leptin/leptin receptor deficient animals exhibit atrophy of lymphoid tissues, particularly the thymus, and such a defect can be reversed by the reposition of the hormoneReference Howard, Lord, Matarese, Vendetti, Ghatei, Ritter, Lechler and Bloom39.
Since malnutrition leads simultaneously to thymic atrophy and hypoleptinaemia, a mechanistic role for insufficient leptin action in the thymus of malnourished subjects was proposedReference Savino40, taking into account that leptin has been shown to prevent starvation-induced thymic atrophyReference Howard, Lord, Matarese, Vendetti, Ghatei, Ritter, Lechler and Bloom39, Reference Mito, Yoshino, Hosoda and Sato41.
At least two mechanisms may play a role in leptin-induced inhibition of thymic atrophy: increase of thymopoiesis and inhibition of apoptosisReference Hick, Gruver, Ventevogel, Haynes and Sempowski42, Reference Mansour, Pereira and Araujo43. Thymopoiesis has been tested in wild-type C57BL/6 and BALB/c mice, in endotoxin-stressed (LPS treated) BALB/c mice, and in leptin deficient obese (ob/ob) miceReference Hick, Gruver, Ventevogel, Haynes and Sempowski42. Leptin is actually able to increase thymopoiesis, but only in leptin-deficient mice or in animals treated with LPS, suggesting that it acts as a thymopoietic factor only in the setting of induced thymic atrophyReference Hick, Gruver, Ventevogel, Haynes and Sempowski42. Although this hypothesis has not been tested in malnutrition models, results obtained from starved animals suggest that a similar phenomenon may occur in the thymus of malnourished subjectsReference Howard, Lord, Matarese, Vendetti, Ghatei, Ritter, Lechler and Bloom39. On the other hand, inhibition of apoptosis has been evaluated in wild-type Wistar rats. Short-term leptin treatment significantly reduces thymocyte apoptosis. This outcome seems to occur predominantly by leptin acting upon CD4+CD8+ cells undergoing maturation, since highest expression of the corresponding receptor is seen at the thymocyte differentiation stageReference Mansour, Pereira and Araujo43. Interestingly, the anti-apoptotic effect of leptin in the thymus is not mediated by the classic JAK2/STAT3 signaling pathway, but rather depends on the activation of the docking protein IRS-1Reference Mansour, Pereira and Araujo43.
Thus, leptin is one possible mediator of malnutrition-induced thymic atrophy. Actually, it is conceivable that under situations of malnutrition, the imbalance between leptin production (which is decreased) and glucocorticoid hormone levels (which are increased) is at least partially responsible for thymocyte depletion and consequent atrophy of the organ (see figure 2). In this respect, it will be worthwhile to test whether this hormone can be applied as a therapeutic tool to partially prevent the harmful effects of malnutrition upon the immune system.
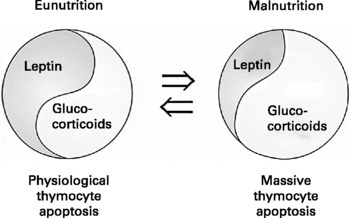
Fig. 2 Putative hormonal circuit involved in triggering the depletion of thymocytes that occurs in malnourished individuals. In physiological conditions there is a balance (herein illustrated by the Chinese Yin/Yang symbol of equilibrium) between glucocorticoids (pro-apoptotic) and leptin (anti-apoptotic), accounting for the normal pattern of thymocyte apoptosis. In malnutrition, there is a decrease in leptin levels, leading to a stimulation of the hypothalamus-pituitary-adrenal axis that results in increased circulating levels of glucocorticoid hormones (upper arrow), which in turn enhance thymocyte apoptosis. As indicated by the bottom arrow, this situation can be reversed by the re-establishment of an appropriate diet (modified fromReference Savino40).
The precise mechanisms responsible for the thymic atrophy seen in acute infections are not completely elucidated, and may vary in distinct diseases. But similar to malnutrition, one major pathway is related to the rise in serum glucocorticoid hormone levels, a classical component of the stress response of the organism to the infection. Thymocyte depletion commonly observed in rabies virus infected miceReference Cardenas-Palomo, de Souza-Matos, Chaves-Leal, Bertho and Marcovistz44 can be prevented by adrenalectomy prior to infection. In murine Chagas disease, we also found high levels of corticosterone, in both acutely and chronically infected animalsReference Corrêa-de-Santana, Paez-Pereda, Theodoropoulou, Gruebler, Nihei, Bozza, Arzt, Villa-Verde, Renner, Stalla, Stalla and Savino45, Reference Leite-de-Moraes, Hontebeyrie-Joskowicz, Leboulanger, Savino, Dardenne and Lepault46. Nevertheless, adrenalectomy alone did not prevent Trypanosoma cruzi-induced cortical thymocyte depletion. More recently, it was demonstrated that complete inhibition of glucocorticoid receptors by in vivo injection of RU-486, did succeed in preventing thymocyte depletion following acute Trypanosoma cruzi infectionReference Roggero, Pérez, Tamae-Kakazu, Piazzon, Nepomnaschy, Besedovsky, Bottasso and del Rey47. Whether such a procedure modifies leptin levels in acutely-infected mice, remains to be determined, and represents an interesting open field of investigation.
The thymic microenvironment in malnutrition and acute infections
In addition to the lymphoid compartment, the thymic microenvironment is affected under various malnutrition and infectious conditions. Morphological changes in the thymic epithelium from protein-malnourished mice include an increase, both in cortical and medullary thymic epithelial cells (TEC), of intracytoplasmic accumulations of large, circular, homogeneously electron-dense profiles, rich in free and esterified cholesterolReference Mittal and Woodward48. Conversely, these lipid-laden epithelial cells cannot exhibit the membrane-bound cytoplasmic vacuoles observed in control animals, and that normally contain the thymic hormone thymulinReference Savino and Dardenne4. By means of morphometry, the volume of the epithelial tissue in the cortex and medulla of thymuses from malnourished mice has been shown to be decreased, as compared to well-nourished control animalsReference Mittal and Woodward48. Unfortunately, no data were reported concerning TEC death in this experimental model.
Decreased thymic endocrine function in malnourished and acutely-infected individuals
One functional parameter that has been evaluated under malnutrition conditions is thymic hormone production by TEC. Protein-malnourished mice exhibit abnormally low levels of circulating thymulinReference Chandra1, Reference Mittal, Woodward and Chandra49, and this is also observed in protein-malnourished rats and humansReference Jambon, Ziegler, Maire, Hutin, Parent, Fall, Burnel and Duheille50. Even in humans suffering from calorie malnutrition secondary to anorexia nervosa, low thymulin serum levels have been reportedReference Wade, Bleiberg, Mosse, Lubetzki, Flavigny, Chapuis, Roche, Lemonnier and Dardenne51. Furthermore, decreased serum thymulin levels have been reported in mice submitted to diets designed to trigger deficiency in zinc, iron, or vitaminsReference Chandra1, Reference Kuvibidila, Dardenne, Savino and Lepault13, Reference Dardenne, Savino, Wade, Kaiserlian, Lemonnier and Bach52,. At least regarding zinc deficiency, similar results have been found in humansReference Prasad, Meftah, Abdallah, Kaplan, Brewer, Bach and Dardenne53. It is noteworthy that the concept of decreased thymic hormone in malnutrition is not restricted to thymulin, since it was also reported for thymopoietin productionReference McDade, Beck, Kuzawa and Adair54. In this study, the authors further showed that prenatal undernutrition was significantly associated with reduced thymopoietin production in interaction with the duration of exclusive breast-feeding. These findings provide support for the importance of foetal and early infant programming of thymic function, and long-term implications for the immune system, and consequently adult disease risk.
In severe infection conditions, thymic endocrine function is also affected. We observed in Trypanosoma cruzi-infected mice a transient decrease in the serum levels of the thymic hormone thymulinReference Savino, Leite de Moraes, Hontebeyrie-Joskowicz and Dardenne55. In human HIV infection a consistent and long-term decrease of thymulin secretion has also been documented, in terms of serum levels and intrathymic contents of the hormoneReference Dardenne, Bach and Safai56–Reference Savino, Dardenne, Marche, Trophylme, Dupui, Pekovic and Bach58.
Increased extracellular matrix in the thymus of malnourished children
In addition to the abnormalities shown in TEC, cells from the thymus of malnourished children exhibit a further microenvironmental alteration, namely, an increase in the deposition of extracellular matrix (ECM) proteins. We studied by histological, ultrastructural, and immunohistochemical means thymuses obtained in necropsies from malnourished children. We observed a consistent increase in the intralobular ECM-containing network, which could be ascertained histologically by the dense reticulin staining. This abnormally dense ECM network contained fibronectin, laminin, and type IV collagen. Importantly, the enhancement of thymic ECM in malnourished individuals positively correlated with the degree of thymocyte depletionReference Lyra, Madi, Maeda and Savino8. This correlation may represent a cause-effect relationship in which the contact of thymocytes with abnormally high amounts of thymic ECM triggers and/or enhances programmed cell death. However, this notion is still hypothetical, demanding experimental demonstration. Similar changes in thymic ECM were observed in glucocorticoid-hormone treated mice and TEC culturesReference Savino and Dardenne4, leading to hypothesis that the enhanced ECM deposition seen in malnutrition may be also related to high levels of serum glucocorticoid hormones. Such an alteration was also seen in acute infections, as exemplified by experimental Chagas diseaseReference Savino, Leite de Moraes, Hontebeyrie-Joskowicz and Dardenne55, Reference Cotta de Almeida, Mendes da Cruz, Bonomo and Savino59. In this infection model, changes in ECM were accompanied by alterations in the migratory response of thymocytes, with abnormal export of CD4+C8+ immature thymocytes, some of them having bypassed the normal selective selection processReference Cotta de Almeida, Mendes da Cruz, Bonomo and Savino59–Reference Savino, Villa-Verde, Mendes-da-Cruz and Silva-Monteiro61. Whether similar cell migration abnormalities exist in malnourished subjects, is still to be elucidated.
Concluding remarks
The various issues discussed above clearly show that the thymus is a common target organ in malnutrition and in acute infections. The changes summarized herein likely have consequences, leading to the impaired peripheral immune response shown in both malnourished and infected individuals. Thus, strategies to promote thymus replenishment should be considered when designing therapeutic approaches, in both malnutrition and acute infectious diseases.
Conflict of interest statement
This work was partially funded with grants from Fiocruz, CNPq and Fapesp (Brazil), CNRS (France). No author has any conflict of interest to declare.





