Although numerous studies have focused on obesity-associated reproductive abnormalities in females, little attention has been paid to male cases, including overall male reproductive health. Recently, more evidence has shown that male obesity is associated with poor semen quality including low sperm concentration and low progressively motile sperm count, lower rate of couple fertility, and low serum total and free testosterone levels(Reference Magnusdottir, Thorsteinsson and Thorsteinsdottir1–Reference Mokdad, Bowman and Ford4). The frequency of erectile dysfunction also increases with increasing BMI(Reference Feldman, Goldstein and Hatzichristou5, Reference Bacon, Mittleman and Kawachi6). All of these problems are contributing factors to male infertility and raise concerns about an alarming trend in North America, since the obese population is increasing. According to Statistics Canada(7), about 33 % of adult Canadians are overweight and 15 % are obese. In the USA, 64 % of adults are overweight, over 30 % are obese and 5 % are extremely obese(Reference Flegal, Carroll and Ogden8).
Testes and sperm have a characteristic lipid composition that is highly enriched in long-chain PUFA (long-chain fatty acids; LCFA), predominantly DPA (22 : 5n-6) in rats and DHA (22 : 6n-3) in humans(Reference Retterstøl, Haugen and Tran9). Although the specific functions of these fatty acids are not known, decreased 22 : 6n-3 in the sperm phospholipid(Reference Zalata, Christophe and Depuydt10) has been identified in infertile men and asthenozoospermic males(Reference Zalata, Christophe and Depuydt10, Reference Tavilani, Doosti and Nourmohammadi11). Infertile men also exhibit a drastic loss of sperm phosphatidylethanolamine (PE) but significant increases of phosphatidylserine(Reference Gulaya, Margitich and Govseeva12). In rats, decreased levels of 22 : 5n-6 are related to smaller testes(Reference Zanetti, Maldonado and Aveldaño13, Reference Furland, Maldonado and Aresti14) and lower fertility(Reference Johnson, Johnson, Gomes and Vandermark15), which could be due to poor spermatid maturation(Reference Davis, Bridges and Coniglio16). These findings indicate that decreased sperm fertility potential is associated with alteration of sperm and testicular lipid composition of n-6 and n-3 LCFA. Whether levels of these fatty acids are altered in obese males are not known.
Dietary n-6 and n-3 fatty acid types have been shown to affect Sertoli cell lipids, the binding capacity of the luteinising hormone receptor in the testis, testosterone production in Leydig cells and reproductive capacity(Reference Blesbois, Douard and Germain17–Reference Marzouki and Coniglio19). In addition, alteration of the content and the ratio of n-6 and n-3 fatty acids in the diet has been found to influence eicosanoid synthesis and metabolism, and affect the fertilising ability in males(Reference Abayasekara and Wathes20). This implies that dietary lipids, such as n-6 and n-3 fatty acids, could be important factors influencing testicular function and reproductive performance. In fact, dietary 22 : 6n-3, more efficiently than arachidonic acid (20 : 4n-6), restored fertility, sperm count and spermiogenesis in 22 : 5n-6- and 22 : 6n-3-deficient Δ-6 desaturase-null mice(Reference Roqueta-Rivera, Stroud and Haschek21). This study indicates the importance of n-3 LCFA, perhaps the balance of n-3 and n-6 LCFA, in fertility, even in the n-6 LCFA-enriched rodent testis.
The Zucker obese (fa/fa) rat has a somatic mutation in the leptin receptor gene and is a widely used animal model for human obesity studies(Reference Corsetti, Sparks and Peterson22). This animal model exhibits many pathological features similar to human obesity such as hyperphagia, hyperinsulinaemia, hyperlipidaemia, but not hyperglycaemia, as seen in the obese Zucker diabetic fatty male rat. By using this animal model, we investigated whether fatty acid compositions in the major phospholipids of testes are affected by the obese condition as well as dietary n-3 LCFA treatment.
Materials and methods
Animals and diets
Male lean (ln; n 12) and obese (fa/fa; n 12) Zucker rats, aged 6 weeks (Harlan, Indianapolis, IN, USA), were randomly assigned to two experimental diet groups (six rats per group) and fed semi-purified diets containing 15 % (w/w) fat for 8 weeks. The basal diet contained (per kg): casein (270 g), maize starch (214 g), dextrose (217 g), cellulose (80 g), vitamin mix (9·5 g), mineral mix (48 g), choline (2·75 g), inositol (6·25 g) and l-methionine (2·5 g) as previously described with a slight increase in maize starch(Reference Field, Ryan and Thomson23). Diets differed in n-3 LCFA level, 0 v. 5 % (w/w of total fatty acids) by using fish oil as the source of n-3 LCFA (Ocean Nutrition Canada, Mulgrave, NS, Canada) (Table 1). The mixture of fat and the fatty acid composition of the diets that were fed are presented in Table 1. Animals were housed in a controlled environment of 21–23°C, 55 % humidity and 14 h light–10 h dark cycles. Animals were allowed access to feed and water ad libitum.
Table 1 Fatty acid composition of the experimental diets
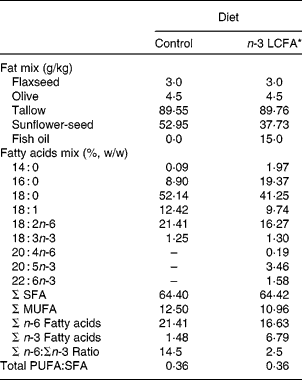
LCFA, long-chain fatty acids.
* Containing both EPA (20 : 5n-3) and DHA (22 : 6n-3).
Animals were euthanised by CO2 asphyxiation and decapitation after a 12 h overnight fast. Body weights, and epididymal, testicular, seminal vesicular and prostate weights were recorded. Excised testes and epididymides were immediately frozen in liquid N2, and stored at − 80°C. Animal care procedures were based on guidelines described in the Canadian Council for Animal Care. The study was approved by the University of Manitoba, Fort Garry Campus Protocol Management and Review Committee.
Caudal epididymal sperm morphology
Caudal epididymis was minced to release the spermatozoa in 2 ml saline (0·9 % NaCl). A sample of minced caudal epididymis was fixed with 200 μl formalin and stained with eosin Y. The sperm morphology for head and tail abnormality was assessed in 200 sperm using 400X conventional light microscopy (Olympus EH, Tokyo, Japan; Sony CCD model XC-711 camera, Tokyo, Japan). Sperm morphology was assessed according to Sprando et al. (Reference Sprando, Collins and Black24) and the Rat Sperm Morphological Assessment Guideline Document(25).
Lipid analysis
Total lipids were extracted from decapsulated testes by the Folch method(Reference Folch, Lees and Sloane Stanley26). Phospholipids were separated on hexane pre-washed silica-gel H-plates (20 × 20 cm) by developing in a prepared tank of chloroform–methanol–2-propanol–0·25 % (w/v) KCl–triethylamine (30:9:25:6:18, by vol.)(Reference Touchstone, Chen and Beaver27). Individual phospholipid bands were visualised with 0·1 % aniline naphthalene sulfonic acid in water (w/v) under UV light. Fatty acid methyl esters were prepared using 14 % BF3 in methanol(Reference Morrison and Smith28).
Fatty acid analysis
Separation of fatty acid methyl esters was performed on a SGE BPX 70 capillary column (35 m × 25 mm internal diameter with 0·25 μm thickness) using a Shimadzu GC17A chromatograph (Mandel Scientific Co. Ltd, Guelph, ON, Canada). H2 was used as a carrier gas at a flow rate of 2·5 ml/min. The temperature programme profile was as follows: 130°C held for 0 min; 10°C/min up to 150°C, held for 0 min; 1°C/min up to 190°C, held for 0 min; 3°C/min up to 240°C, held for 15 min. Injection temperature was 275°C, and detector temperature was 320°C. All fatty acids were compared with a commercial standard (NuChek Prep 461).
Statistical analysis
The effect of genotype and diet on sperm morphology and phospholipid fatty acid composition in testes and epididymides was analysed by two-way ANOVA using statistical software (SAS version 9.1; SAS Institute, Inc., Cary, NC, USA). Significant effects of treatment were defined using Duncan's multiple-range test(Reference Steel, Torrie and Dickey29). All data are expressed as mean values and standard deviations.
Results
Body and organ weights and sperm morphology
Genotype differences were found in body and organ weights between the lean and obese animals. The obese rats in the control and n-3 LCFA groups had a significantly higher body weight, 42·5 and 27·4 %, respectively, than the lean animals fed the same diet, as was expected (Table 2). In both absolute (g) and relative (% of body weight) weight, the obese rats had significantly smaller paired epididymides and seminal vesicles, but larger prostate glands in comparison with their lean counterparts (P < 0·0001) (Table 2). There were no differences in the absolute weight of paired testes, but a significantly lower relative testicular weight (P < 0·001) was observed in obese rats. These body and organ weights were independent of dietary treatment. Testes and reproductive accessory organs are shown in Fig. 1. Interestingly, the obese rats had noticeably shorter epididymides. It was found that 35 % of lean rats and 60 % of obese rats showed differences in each testes pair, with a range of 1·2- to 2·7-fold more in the larger testes than the underdeveloped testes (data not shown).
Table 2 Effect of dietary n-3 long-chain fatty acids (LCFA) on organ weight of lean and obese Zucker rats
(Mean values and standard deviations for six rats per group)

a,b,c Mean values within a row with unlike superscript letters were significantly different by multiple comparison (P < 0·05).
* Significant effects of genotype and diet were identified by two-way ANOVA.
† Paired weights.

Fig. 1 Reproductive organs and accessory glands of lean and obese Zucker rats: (a) testes; (b) prostate glands; (c) epididymides; (d) seminal vesicles. The dotted line adjacent to the epididymis of the lean animal represents the caudal epididymis removed for sperm morphology analysis. Pictures were taken from 14-week-old Zucker rats (lean body weight, 284 g; obese body weight, 407 g).
Overall sperm integrity was not affected by genotype or diet. More than 50 % of sperm from obese rats were morphologically abnormal in both diet groups but no differences were found for the lean rats. This might be due to a large variation in sperm integrity, for example, the normal sperm in obese rats fed the n-3 LCFA diet ranged from 0 to 153 among 200 sperm counted. The obese rats on the n-3 LCFA diet had fewer tail abnormalities but more head abnormalities (16·4 (sd 12·5) and 23·0 (sd 22·5) %, respectively) than the obese rats fed the control diet (33·9 (sd 17·7) and 4·2 (sd 2·6) %, respectively). Head abnormalities were identified as headless sperm, sperm with bent necks, or pinheads. Tail abnormalities included bent or coiled tails.
Fatty acid composition in testicular phospholipids
Fatty acid composition was determined in phosphatidylcholine (PC) and PE, since these are the most abundant phospholipids found in the testis.
Phosphatidylcholine
Both genotype and diet significantly altered the fatty acid composition in PC (Table 3). Obese rats had higher levels of dihomo-γ-linolenic acid (20 : 3n-6) and total MUFA, but lower 20 : 4n-6 compared with lean rats. The n-3 LCFA diet significantly elevated myristic acid (14 : 0), 16 : 1, linoleic acid (18 : 2n-6), 20 : 3n-6 while decreasing 20 : 4n-6, adrenic acid (22 : 4n-6) and total SFA in comparison with the control diet. The level of 22 : 6n-3 was also higher in both lean and obese animals after being fed the n-3 LCFA diet. The acylation of dietary n-3 LCFA into 22 : 6n-3 was 2·4 times higher in obese rats than in the lean animals fed the same diet. Tetracosapentaenoic acid (24 : 5n-3) and tetracosahexaenoic acid (nisinic acid; 24 : 6n-3) were also higher in obese rats fed the n-3 LCFA diet. Overall, the n-3 LCFA diet significantly decreased the total n-6:n-3 fatty acids ratio in testicular PC. The predominant LCFA in testicular PC were 20 : 4n-6 (11·5–14·6 %, w/w) and 22 : 5n-6 (12·3–14·6 %, w/w) (Table 3).
Table 3 Effect of dietary n-3 long-chain fatty acids (LCFA) on fatty acid composition of phosphatidylcholine in the testes of lean and obese Zucker rats
(Mean values and standard deviations)

nd, Not detected.
a,b,c Mean values within a row with unlike superscript letters were significantly different by multiple comparison (P < 0·05).
* Significant effects of genotype and diet were identified by two-way ANOVA.
Phosphatidylethanolamine
The genotype effect on fatty acid composition in PE was minor. Obese animals had higher 22 : 6n-3 and 24 : 6n-3 and lower 22 : 5n-6 than their lean counterparts. The genotype differences became evident when animals were fed the n-3 LCFA diet by further elevating 22 : 6n-3 and 24 : 6n-3 while decreasing 22 : 5n-6 in obese rats in comparison with lean rats fed the same diet. Rats fed the n-3 LCFA diet had higher levels of 18 : 2n-6, 20 : 3n-6, 22 : 5n-3 and tetracosapentaenoic acid (24 : 5n-6) but lower 20 : 4n-6 and 22 : 4n-6 in comparison with those fed the control diet. Similar to PC, the acylation of dietary n-3 LCFA into 22 : 6n-3 was 1·6 times higher in obese rats than in the lean rats fed the same diet. This resulted in a significant low total n-6:n-3 fatty acids ratio in PE. The major LCFA in PE were also 20 : 4n-6 (20·3–22·5 %, w/w) and 22 : 5n-6 (20·6–26·2 %, w/w) (Table 4).
Table 4 Effect of dietary n-3 long-chain fatty acids (LCFA) on fatty acid composition of phosphatidylethanolamine in the testes of lean and obese Zucker rats
(Mean values and standard deviations)

nd, Not detected.
a,b,c Mean values within a row with unlike superscript letters were significantly different by multiple comparison (P < 0·05).
* Significant effects of genotype and diet were identified by two-way ANOVA.
Fatty acid composition in underdeveloped testes
The fatty acid composition of underdeveloped testes was compared with that of normal-size testes. Among those analysed for fatty acids, only one or two rats per diet group showed over 30 % differences in size between the paired testes; the underdeveloped testes were grouped together regardless of diet and genotype. Thus the following data present underdeveloped (n 4) and normal (n 18)-size testes using a typical gas chromatogram (see Fig. 2). The underdeveloped testes had characteristically low (P < 0·0001) 22 : 5n-6 in both PC (72 % less; 3·9 (sd 3·3) %) and PE (71 % less; 6·8 % (sd 2·4) %) when compared with normal-sized testes (13·7 (sd 2·0) and 24·2 (sd 2·6) % in PC and PE, respectively). However, 20 : 4n-6, the precursor of 22 : 5n-6, was significantly (P < 0·0005) increased in PC (13·1 (sd 1·4) and 18·3 (sd 5·0) % in normal and underdeveloped testes, respectively). Compared with the level of 22 : 5n-6 in PC of normal-size testes in Table 3, the level in the underdeveloped testes of the lean control (n 1), obese control (n 2) and obese n-3 LCFA (n 1) groups was only 8·8, 2·3 and 2·3 %, respectively. The level of 22 : 5n-6 in underdeveloped testes PE in the lean control (n 1), obese control (n 2) and obese n-3 LCFA (n 1) groups was also 9·0, 7·8 and 8·0 %, respectively, much lower than the level in normal-size testes (Table 4). The underdeveloped testes also had higher stearic acid (18 : 0) in both phospholipid fractions (data not shown). The level of 22 : 6n-3 was also low in PC and PE, but a significant difference was not found.

Fig. 2 A typical chromatogram showing the content of arachidonic acid (20 : 4n-6) and DPA (22 : 5n-6) in phosphatidylcholine in normal and underdeveloped testes.
Discussion
Effect of genotype and dietary n-3 long-chain fatty acids on organ weights and sperm morphology
The present study found that the sizes of male sexual and accessory organs were affected by obesity. Obese Zucker rats had significantly smaller and shorter epididymides and seminal vesicles, but larger prostate glands. Obese rats had abundant unbalanced paired testis weights, leaving one testis underdeveloped. Both head and tail abnormalities in caudal epididymal sperm were also observed in obese rats. While there was no significant difference in overall sperm morphology between obese and lean animals, the n-3 LCFA diet decreased tail abnormalities but increased head abnormalities in obese rats. We noted a large variation in sperm morphology between rats in each diet group in this animal model; a larger sample size would confirm the effects of diet on sperm integrity. Although not measured in the present study, investigating sperm motility could perhaps explain its relationship with the epididymal length, since the spermatozoas undergo the maturation process to acquire motility function while moving progressively from caput, corpus, then to the caudal epididymis region. n-3 LCFA supplementation at the level of 5 % (w/w, total fatty acids) did not affect male sexual and accessory organ weights.
Effect of genotype and dietary n-3 long-chain fatty acids on fatty acid compositions in phospholipids in testes
The present study identified that there were no major differences in the fatty acid composition in phospholipids between lean and obese rats fed the control diet. Regardless of the genotype, the most quantitatively abundant LCFA found in the testes were 20 : 4n-6 and 22 : 5n-6 in PC (27–28 %) and PE (46–48 %), showing an n-6-enriched environment in the testes of rats. This finding has been confirmed by others(Reference Furland, Maldonado and Aresti14) and indicates that these fatty acids are minimally affected by the obese condition.
In response to dietary n-3 LCFA, both lean and obese rats increased the levels of n-3 fatty acids, especially 22 : 6n-3, in testes at the expense of major n-6 fatty acids. This suggests that a competition for metabolic enzymes between n-6 and n-3 fatty acids, with preformed n-3 fatty acids being the preferred substrate for metabolism and incorporation into tissue membranes, also occurred in the testis as found in other tissues(Reference Blesbois, Douard and Germain17, Reference Sebokova, Garg and Wierzbicki18, Reference Retterstøl, Haugen and Christophersen30, Reference Chanmugam, Boudreau and Hwang31). Our findings demonstrate that the acylation of dietary n-3 fatty acids into testicular 22 : 6n-3 was about 2-fold higher in obese Zucker rats in comparison with lean rats, suggesting that dietary n-3 manipulation has the greater effect in the obese condition. Guerre-Millo et al. (Reference Guerre-Millo, Guesnet and Guichard32) reported that plasma and intracellular membranes of adipocytes in obese Zucker rats decreased 2-fold in the n-6:n-3 fatty acids ratio mainly due to an enrichment in 22 : 6n-3 after treatment with a 22 : 6n-3-containing diet. It may be that direct acylation of 22 : 6n-3 into phospholipids is more active in obese Zucker rats, which requires further experimentation. It can also be postulated that our finding may be related to altered haemodynamics in testes in the obese condition, since blood flow through epididymides and testes was elevated in obese Zucker rats when expressed on a per g tissue basis(Reference West, Prinz and Francendese33). This is an interesting finding considering that obese males have more risk factors contributing to male infertility(Reference Magnusdottir, Thorsteinsson and Thorsteinsdottir1–Reference Mokdad, Bowman and Ford4, Reference Lima, Cavaliere and Knobel34). Overall, the study findings indicate that obese males with infertility problems may respond effectively to dietary lipid treatments and future studies need to address this in human subjects. Dietary intervention may have potential as an effective treatment for male reproductive problems by altering the major fatty acids for reducing infertility in oligo- and/or asthenozoospermic men(Reference Aksoya, Aksoy and Altınkaynak35).
DPA and underdeveloped testes and epididymides
Although it is preliminary because of limited numbers measured, the present study found that the underdeveloped testis had dramatic decreases of 22 : 5n-6 in major phospholipids. A current ongoing study in our laboratory with older Zucker obese rats also shows this phenomenon in the testis (M Suh, unpublished results). While not knowing if this decrease is the result or cause of testis underdevelopment, a similar selective decrease of 22 : 5n-6 was also identified in smaller rat testes, which were treated with doxorubicin, an antineoplastic drug, or had undergone surgery for cryptorchidism(Reference Zanetti, Maldonado and Aveldaño13, Reference Furland, Maldonado and Aresti14). The fatty acid 22 : 5n-6 is known to be germ cell-specific and associated with spermatid maturation(Reference Davis, Bridges and Coniglio16, Reference Robinson, Johnson and Poulos36). Thus a decrease in this fatty acid is related to germ cell deficiency. Due to limited sample numbers, the underdeveloped testes were all grouped together regardless of diet and obese condition; thus, further confirmation is required of whether n-3 LCFA are metabolised similarly in normal-size testes and improve or worsen the testicular function. Since the precursor fatty acid, 20 : 4n-6, was increased, the enzymes involved in 22 : 4n-6 conversion to 22 : 5n-6 could be blocked in the testes of this animal model. According to Sprecher's pathway(Reference Voss, Reinhart and Sankarappa37), Δ-6 desaturase enzyme may be responsible for this, thus further study is needed. Obese Zucker rats have low serum testosterone levels at age 2, 3 and 4 months compared with their lean counterparts(Reference Young, Frink and Longcope38). Whether 22 : 5n-6 is also involved in testicular testosterone production requires further investigation.
There is limited research in relation to the effects of obesity and dietary responses on male reproductive health. The present study provides experimental evidence that the lipid microenvironment of phospholipids in the testes of obese rats can be efficiently modulated by dietary fat intervention. The clear benefits of n-3 LCFA in rats v. human subjects require further study. Considering Coniglio et al. (Reference Coniglio, Grogan and Rhamy39), postulation that 22 : 6n-3 serves a function in the human testis similar to that served by 22 : 5n-6 in the rat testis, dietary manipulation of n-6 or n-3 fatty acids and the resulting outcome found in rats can be translated to humans. The obese Zucker rat provides interesting characteristics in sex and accessory organs, especially unbalanced paired testis development. Whether germ cell populations are increased in proportion to the level of 22 : 5n-6 needs further investigation. Hormonal changes not investigated in the present study merit exploration in future studies as well. The Zucker (fa/fa) rat could be a useful model for further study on mechanisms involved in testis development and male fertility in the obese state. Overall, dietary fat can be an important determinant in male reproductive health.
Acknowledgements
The present study was supported by the Natural Sciences and Engineering Research Council of Canada and Manitoba Medical Service Foundation, and Agri-food Research Development Initiative. The authors appreciate the technical assistance of Mr Dennis Labossiere and thank Dr Catherine Field at the University of Alberta for the formula for the fat mixture and Dr Peter Zahradka for allowing us to take tissues from their study.
M. S. was responsible for overall data acquisition, analysis, interpretation and manuscript preparation. K. J. M. measured sperm morphology and contributed to the statistical analysis. A. D. contributed to fatty acid analysis. C. G. T. designed the nutritional intervention and contributed to animal care.
There are no conflicts of interest in the present study.








