Mild cognitive impairment (MCI) is a notable concern among older adults. A meta-study conducted on individuals aged 50 years and older unveiled that the worldwide prevalence of MCI in community-dwelling populations exceeds 15 %(Reference Bai, Chen and Cai1). According to the estimation of the WHO and the World Alzheimer Report 2021 from Alzheimer’s Disease International, approximately 55 million people are living with dementia, which is projected to increase to 78 million in 2030 and 139 million in 2050(Reference Gauthier, Rosa-Neto and Morais2,3) . MCI represents the intermediate stage between the cognitive changes associated with normal ageing and the more severe cognitive decline seen in dementia, including Alzheimer’s disease(Reference Petersen4).
Along with rapid socio-economic transformation, changes in dietary patterns have been accompanied by considerable disease burdens. The findings, led by GBD 2017 Diet Collaborators, suggest that the high intake of Na was one leading dietary risk factor, and China had the highest rates for some diet-related diseases among the world’s twenty most populous countries in 2017(Reference Afshin, Sur and Fay5). Specifically, the existing studies concerning the association between dietary patterns and cognitive function were focused on the plant diary(Reference Zhu, Chen and Shen6,Reference Lourida, Soni and Thompson-Coon7,Reference Psaltopoulou, Sergentanis and Panagiotakos8,Reference Boumenna, Scott and Lee9,Reference Morris, Tangney and Wang10) . These studies confirmed that high adherence to the Mediterranean diet is associated with a lower risk of cognitive decline(Reference Lourida, Soni and Thompson-Coon7,Reference Psaltopoulou, Sergentanis and Panagiotakos8) .
Although the link between dietary factors and health has been extensively studied, maintaining high adherence to a healthy diet is often difficult to implement in people’s daily lives. One potential reason is that food is not just an essential need for energy supply but is also related to people’s subjective taste preferences and choices based on their profound cultural background as well as social and psychological satisfaction(Reference Sorokowska, Pellegrino and Butovskaya11). Sweet, sour, salty, hot and bitter are five fundamental parts that constitute the basic taste in Chinese food history. As an important guide for food choice, taste plays a central role, especially for older adults who are not very sensitive to food energy contents and ingredients and have relatively little knowledge of nutrition. The harmony of taste can not only improve food enjoyment but also promote health. Individual taste preference is a pivotal predictor of nutrition intake(Reference Louro, Simões and Castelo12). However, studies on taste preference and its relationship with cognitive impairment in China are still uncertain. Thus, we aimed to: (1) analyse the association between taste preferences and MCI among older adults with different sociodemographic features; (2) explore potential moderating mechanisms by sex, living areas, education, exercise frequency and geographical regions; and (3) examine whether cardiometabolic diseases (CMD) can affect taste preference-related cognitive decline. The findings will provide a perspective on cognitive impairment preventive intervention starting from a more familiar dietary concept of older adults to preserve cognitive function.
Materials and methods
Study design and participants
Data used in this study were obtained from the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The CLHLS was initiated in 1998 and applied a multistage, stratified cluster sampling covering the twenty-three provinces in China. To date, eight rounds of surveys have been carried out in 1998, 2000, 2002, 2005, 2008, 2011, 2014 and 2018. The participants included older adults aged 65 years and older.
Our study used the 2008–2018 wave of the CLHLS. The study includes older adults aged 65 to 90 years recruited in 2008 and followed in 2011, 2014 and 2018. At entry, we excluded participants with cognitive impairment, younger than 65 years or older than 90 years of age, as the prevalence of MCI increased with age(Reference Bai, Chen and Cai1) and there may be survival bias for older people. Additionally, we excluded people with MCI at baseline (2008) or data that were not available for specific taste preferences. Finally, 6423 participants were involved in the final analysis (Fig. 1).
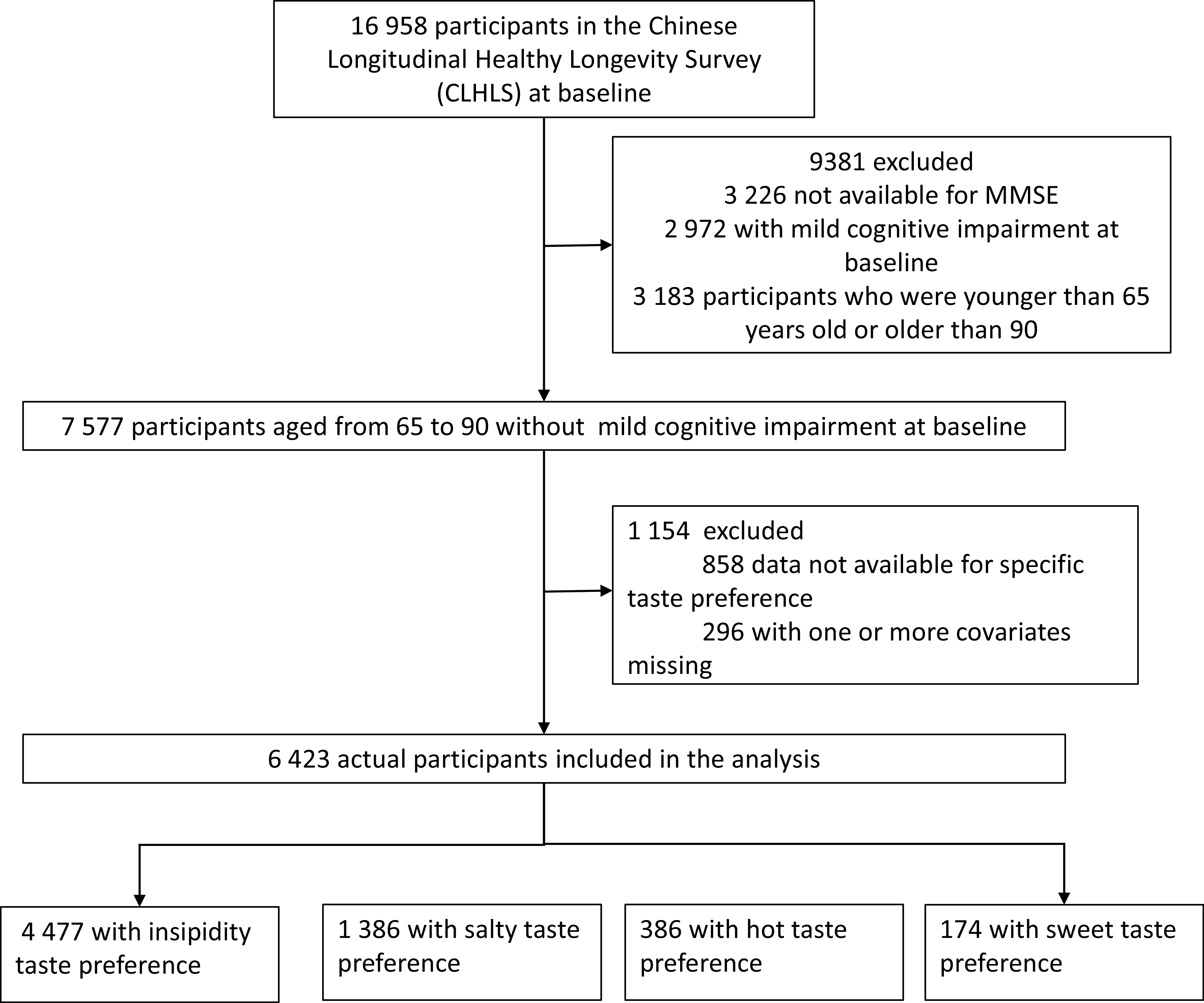
Fig. 1. Flow chart of samples. MMSE, Mini-Mental State Examination.
Exposure: taste preference assessment
The main exposure was the taste preference based on self-report. The taste preference was based on self-judgement and basic notions of health and illness from experience. Thus, it might be a comprehensive indicator of the dietary habits of older adults, which can help us judge the unique health effect of diet for individuals, although the perception and taste threshold varies among people(Reference Dora, Lim and Haron13).Notably, cognitive function and taste preference may exert mutual influence. A study demonstrated that in individuals with Parkinson’s disease, MCI had a detrimental effect on the ability to identify sour and salty tastes(Reference Cecchini, Federico and Zanini14). Moreover, the disruption of sweet taste receptor signalling could potentially impede cognitive functions, stemming from hypothalamic dysfunction and hormonal activity alterations, ultimately impacting critical cognitive brain regions(Reference Welcome, Mastorakis and Pereverzev15). Therefore, when assessing the influence of taste preferences on MCI, caution is warranted, as self-reported taste preferences may be subject to interference from cognitive functions.
The participants’ taste preferences were assessed by a self-reported question: ‘What kind of flavour do you mainly have?’ (insipidity, salty, sweet, hot and crude). Taste insipidity denotes a preference for dishes that are subtly seasoned, allowing the natural flavours of the ingredients to predominate. Consequently, we classified responses into four categories: insipidity (encompassing crude), salty, sweet and hot. Participants were then grouped into four cohorts based on their latest taste preference, with those favouring insipidity serving as the reference group. Considering that the sensitivity of taste preferences tends to decrease with age(Reference Sergi, Bano and Pizzato16), individuals with strong taste preferences may mistakenly categorise their preferences as ‘insipid’. This classification method could potentially underestimate the influence of taste preferences.
Cognitive function assessment
The CLHLS used the Mini-Mental State Examination (MMSE) to measure cognitive function. The MMSE used in the CLHLS has been adapted to the Chinese language and socio-economic context(Reference Yi and Vaupel17). As the MMSE scale does not need to rely on special measuring instruments and special professional training, it has high reliability and validity and is still widely used in the screening of cognitive impairment and dementia(Reference Folstein, Folstein and McHugh18,Reference Ramlall, Chipps and Bhigjee19,Reference Mitchell20) . Specifically, the MMSE has a Pearson correlation coefficient of 0·887, and the retest reliability remains high (0·827) after 24 h(Reference Folstein, Folstein and McHugh18). A survey of older adults over 60 years of age confirmed that the MMSE scale had a sensitivity of 63·6 % and specificity of 76·0 %(Reference Ramlall, Chipps and Bhigjee19).
In this study, MCI was categorised as a dummy variable according to the MMSE score. To control the effect of education, we use the cut-off points of 18, 21 and 25 for people without formal education, with primary school education (1–6 years) and higher education (>6 years), respectively(Reference Zhu, Chen and Shen6). In addition, 50·09 % (3217) of participants were illiterate, 35·05 % (2251) had 1–6 years of education and 14·87 % (955) had more than 6 years of education. The total number of participants in the study was 6423. The analyses were restricted to the first instance of cognitive impairment.
Covariates
Previous research has shown that there are many known risk factors for MCI, which can be roughly classified into three parts. First are the lifestyle-related risk factors. Previous studies show that the risk of cognitive impairment increased with people who have smoked(Reference Shi, Zhang and Yue21), drank alcohol frequently(Reference Anttila, Helkala and Viitanen22) and were physically inactive(Reference Borelli, Leotti and Strelow23). Second, related to health status, the existing evidence shows that obesity and diabetes significantly increase the risk of AD(Reference Profenno, Porsteinsson and Faraone24) and cerebral vessel disease(Reference Arvanitakis, Capuano and Leurgans25), and midlife vascular risk factors are also important predictors of incident dementia(Reference Gottesman, Schneider and Zhou26). Third, considering sociodemographic factors, people with a higher level of educational attainment(Reference Petersen, Roberts and Knopman27), those not single(Reference Guaita, Vaccaro and Davin28) and those living in urban areas(Reference Nunes, Silva and Cruz29) were less likely to develop dementia.
Based on the above studies, we included time-varying covariates, including age (in years; continuous score), marriage status (in marriage or not), regular exercise (yes or no, accessed by the question ‘Do you do exercise regularly at present’), smoking now (yes or no, accessed by the question ‘Do you smoke at the present time’), alcohol consumption (yes or no, accessed by the question ‘Do you drink alcohol at the present time’), overweight (BMI ≥ 24, defined by the China CDC), self-rated health status (bad, so so and good), and activities of daily living (ADL, categorised into quartiles of independent, mildly dependent (1–2 items cannot be completed independently), moderately dependent (3–4 items) and very dependent (more than four items)). Existing evidence has proven that cardiometabolic disorders influence subsequent cognitive function(Reference Kesse-Guyot, Julia and Andreeva30). Therefore, we included five CMD, including hypertension, diabetes, heart disease, cerebrovascular disease and dyslipidaemia, as covariates, accessed through a self-reported question: ‘Do you currently have any of the following conditions?’
The time-stable covariate included some demographic variables, including sex (male or female), residence area of the interviewee (urban or rural), education (educated or illiterate), ethnic group (Han or mionrity) and geographical regions by representative taste preference (salty areas, sweet areas or hot areas). As taste preference varies across China’s vast territory due to its close relationship to the climate, culture and local consumption levels(Reference Baharuddin and Sharifudin31), in specific regions, one or two taste preferences usually play the dominant role. We use the indicator geographical regions by their representative taste preference to capture the macro-influence of the environment. The geographical location at the province level was categorised into three groups: spiciness areas (central and south China), salt areas (northern coastal areas and northern China) and sweet areas (eastern China)(Reference Song32).
Statistical analysis
In descriptive statistics, continuous variables are presented as the mean and standard deviation, while for categorical variables, the frequency and proportion are listed. We used the independent-samples t test for continuous variables and the χ 2 test for categorical variables to compare the baseline characteristics of individuals by whether they developed MCI or not.
We calculated Cox proportional hazards models with MCI as the outcome. The people who did not develop cognitive impairment in their last survey were defined as censored. The prevalence of MCI increases with age(Reference Petersen, Roberts and Knopman27,Reference Petersen, Lopez and Armstrong33) , and from the age of 65 years, the incidence of developing cognitive impairment increases rapidly. As the participants with MCI before the baseline survey would not have been present for this study, they were not observationally at risk of developing MCI before the initial survey (2008). To solve this, we removed the individual from the risk set between the point of origin and the time of the initial contact by using PROC PHREG in SAS(Reference Allison34). We employed the STATA command ‘stptime’ to calculate incidence rates per 1000 person-years.
The survey was repeated approximately every 3 years from 2008 to 2018. Considering that some covariates may change throughout the observation, we used a 3-year lag-off to incorporate time-dependent covariates. For the time-stable variables, we used the data from the first time they were available (2008) in the survival analyses. Considering that dietary patterns are closely related to geography, lifestyle and other characteristics(Reference Baharuddin and Sharifudin31), taste may play a different role among people from distinct areas or with different lifestyles. Additionally, considering that cognitive function declined at a faster rate with an increasing number of CMD(Reference Jin, Liang and Hong35), we conducted subgroup analyses based on no CMD, one CMD and coexisting CMD (having at least two). Therefore, we divided the samples according to several characteristics and modelled them separately to test the potential heterogeneity. The characteristics included sex (female or male), residence areas (urban or rural), education (educated or illiterate), frequent exercise (yes or no), CMD (without CMD, one CMD and two CMD) and geographical regions by taste preference according to traditional Chinese experience (salty areas included Liaoning, Jilin, Heilongjiang, Beijing, Tianjin, Hebei, Shanxi, Henan, Hainan and Shanxi; sweet areas included Shanghai, Jiangsu, Zhejiang, Anhui, Fujian, Jiangxi, Shandong and Guangdong; hot areas included Chongqing, Sichuan, Hubei, Hunan and Guangxi).
We conducted several sensitivity analyses to verify the robustness of the results. First, we applied the competing risk model to compare the cause-specific hazard ratios (HR) for each pair of taste preference groups. Then, we defined an MMSE score ≤18 as MCI without educational adjustment. Furthermore, we used the propensity score weighting method to balance the distribution of other confounding variables at baseline (online Supplementary Table 2). Considering the potential bias stemming from subjective taste preference, we incorporated objective dietary patterns related to salt-preserved vegetables, sugar and garlic, which roughly correspond to preferences for salty, sweet and hot tastes, respectively (online Supplementary Table 3).
In our analysis involving all the participants, we first computed the unadjusted HR along with its 95 % CI in the Cox model without considering any covariates. Subsequently, we introduced confounding variables to derive the adjusted estimates. These identical confounding variables were also included in our subsample analyses. In this study, statistical significance was determined as a two-sided P value less than 0·05. The statistical analyses were performed using STATA 15 and SAS Studio 3.8 statistical software(36). Statistical significance was defined as a two-sided P value less than 0·05.
Results
The baseline characteristics of the 6423 participants are presented in Supplementary Table 1. Their mean age at baseline was 78·21 (sd = 7·65), and 51·60 % were male. In addition, 50·09 % of the participants were illiterate, 53·15 % were in marriage and most of them lived in rural areas (58·03 %). The majority of participants were in good health: only 18·92 % of participants were obese, only 14·51 % rated themselves as having poor health status and 95·48 % were ADL-independent.
Among the 6423 participants included in the analysis, 2534 (39·45 %) developed MCI during the follow-up by 2018. The incidence of MCI was 63·12 per 1000 person-years (95 % CI (60·71, 65·63)). Starting from the time of investigator entry into the study, the median survival time was 6·25 years (95 % CI (6·17, 6·33)). Compared with people with normal cognitive function throughout the survey, those who developed cognitive impairment were more likely to be older (80·84 v. 76·50), female (50·71 % v. 46·90 %; P < 0·005), illiterate (57·02 % v. 45·56 %; P < 0·001), not in marriage (56·27 % v. 40·70 %; P < 0·001), physically inactive (65·19 % v. 59·89 %, P < 0·001), and had a healthier lifestyle, better self-rated health status and a higher proportion of independent ADL. The structure of ethnic groups, residence areas and chronic disease did not differ significantly.
Supplementary Fig. 1 shows the link between taste preference and MCI. In China, insipidity was the most important taste preference (69·70 %), and salt was the second (21·58 %). Although the insipidity and salty tastes were the dominant taste preference in all three geographical regions by taste preference, sweetness was the third dominant taste preference in the sweet region, and hotness was also the third dominant taste preference in the hot region. Sweet-liking people had the highest proportion of developing MCI (50·26 %), while the proportion of participants with a salty taste developed MCI the lowest (35·50 %). The incident rates for insipidity, salty, sweet and hot taste preference were 62·58 per 1000 person-years (95 % CI (59·72, 65·57)), 54·68 (95 % CI (50·06, 59·74)), 95·00 (95 % CI (82·53, 109·36)) and 90·06 (95 % CI (72·99, 111·12)), respectively.
In the full model (Fig. 2), compared with insipidity taste preference, salty taste was associated with a lower risk of developing poor cognitive function (adjusted HR 0·83 (95 % CI (0·76, 0·90)), while sweet (1·39, 95 % CI (1·22, 1·59)) and hot tastes (1·20 (95 % CI (1·02, 1·42)) were risk factors after adjusting for covariates. This pattern was also confirmed in all three sensitivity analyses. We observed a similar pattern in the analysis by the competing risk model, the model with alternative cognitive function measurement and the model with propensity score weighting (online Supplementary Table 2). The protective effect of salt remains significant despite the consumption of salt-preserved vegetables. Similarly, the detrimental impact of sweet taste persisted regardless of frequent sugar intake. Moreover, spiciness preference was found to adversely affect cognitive function among older adults who frequently consumed garlic. However, the association between spiciness and MCI was statistically insignificant in older adults who did not often consume garlic. These findings collectively indicate that taste itself still plays a central role in cognitive function, even when objective dietary patterns are considered (online Supplementary Table 3).
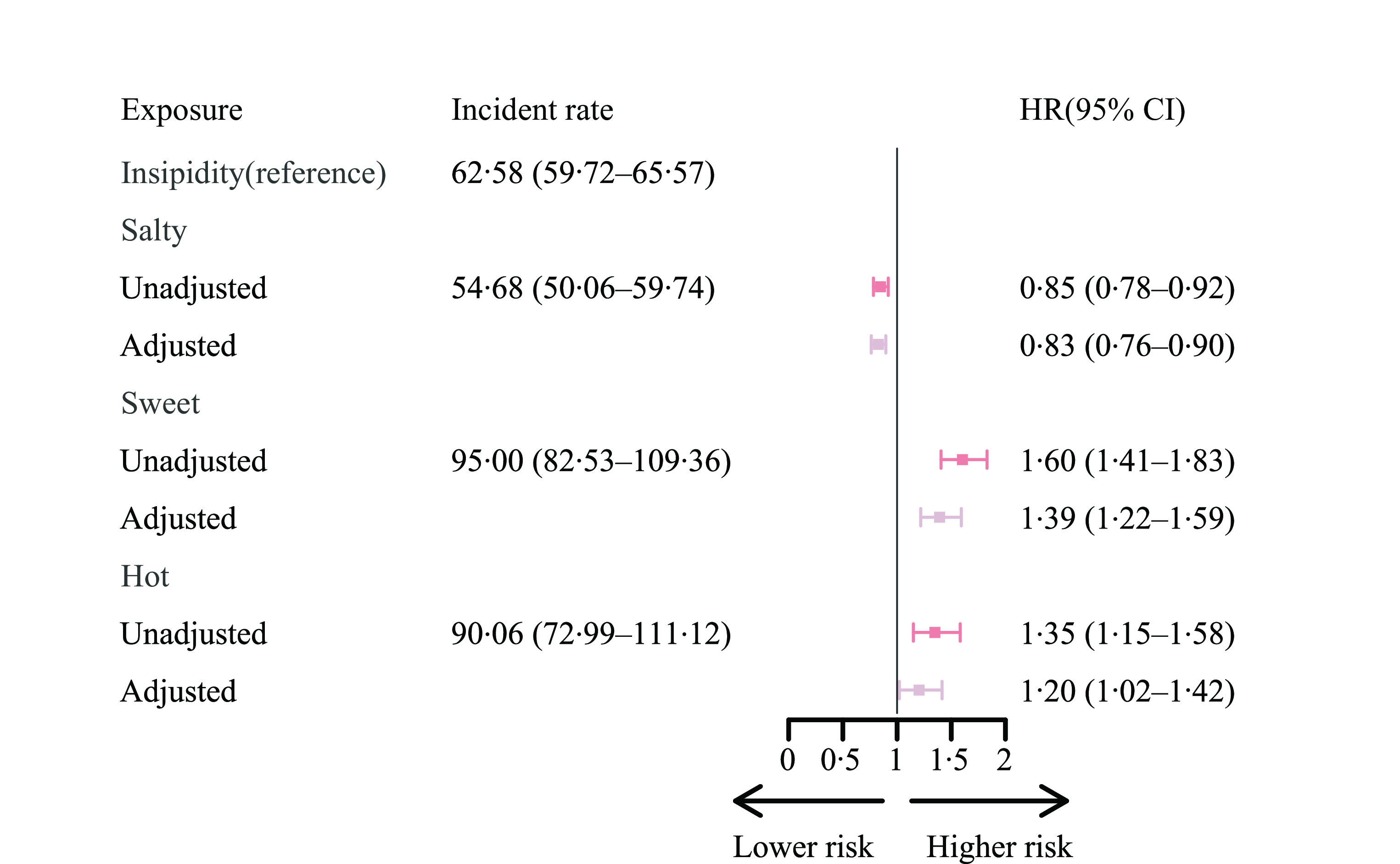
Fig. 2. The risk of developing MCI among people with different taste preferences for Chinese older adults. (1) The unadjusted regression model just included taste preferences and the adjusted regression models were multivariable-adjusted for age (years), sex (male or female), illiterate (yes or no), marriage (in marriage or not), ethnic group (Han or minority), urban/rural residence, geographic region by representative taste preference (salty areas, sweet areas or hot areas), regular exercise (yes or no), smoke now (yes or no), often drink alcohol (yes or no), overweight (yes or no), self-rated health status (bad, so so and good), ADL (independent, mild dependent, moderate dependent and very dependent), and cardiometabolic diseases including hypertension (yes or no), diabetes (yes or no), heart disease (yes or no), cerebrovascular disease (yes or no), and dyslipidemia (yes or no). (2) We utilised the STATA command stptime to calculate incidence rates per 1000 person-years. (3) ADL, activities of daily living; MCI, mild cognitive impairment.
When the analyses were stratified by sex, living area, education and exercise frequency (Fig. 3), a similar significant protective effect by salty taste and a deleterious effect by sweet taste were still prominent. No significant interaction was identified across sex and exercise frequency. However, the detrimental effect of sweet taste was intensified among urban residents (1·46, 95 % CI (1·21, 1·76)) compared with that in the rural group (1·18, 95 % CI (0·96, 1·44)). For illiterate participants, the hot-liking people were more likely to develop MCI (1·81, 95 % CI (1·44, 2·27)), while the effect was not statistically significant for educated people.
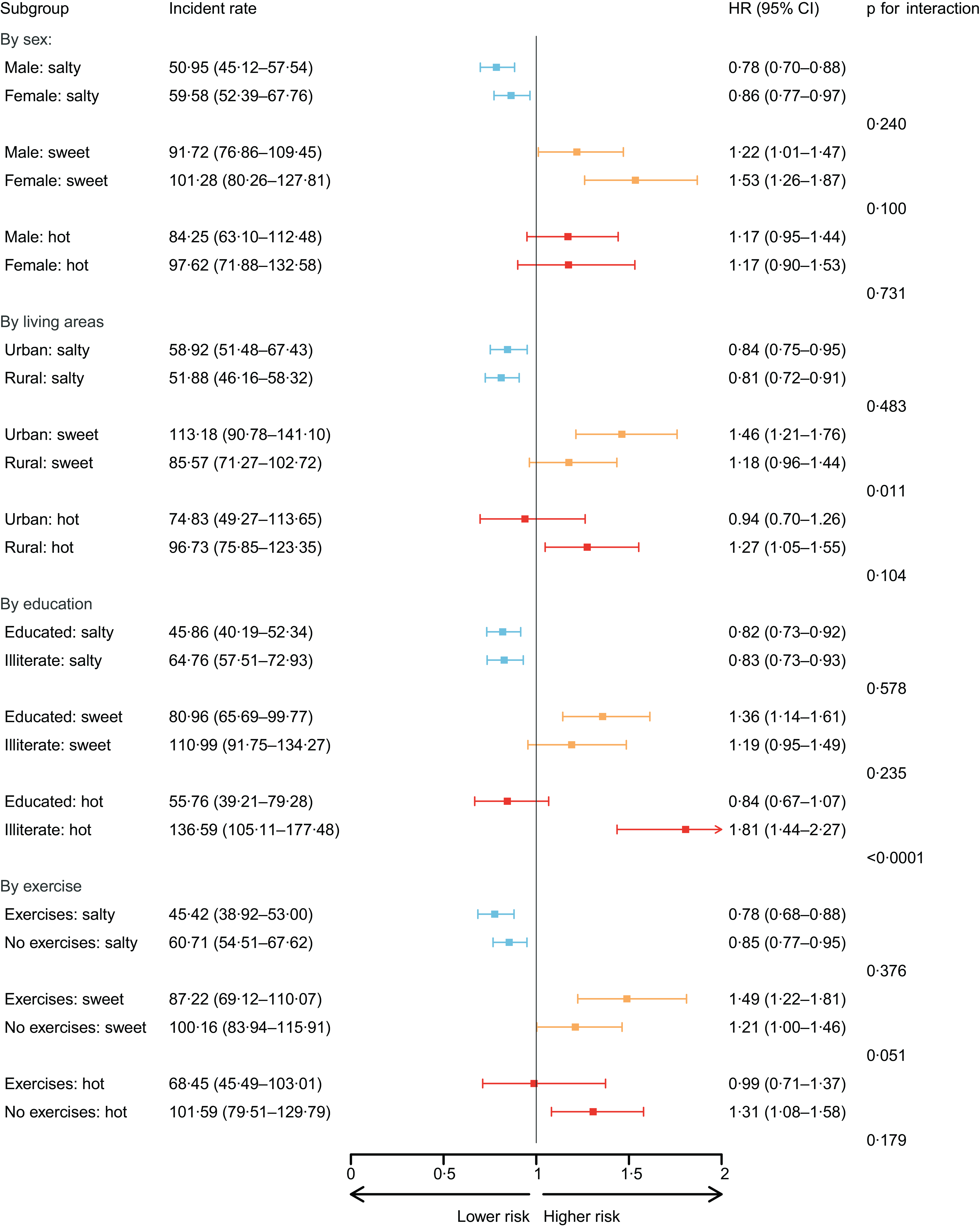
Fig. 3. The effect of taste preference for participants across sex, living areas, education and exercise frequency. (1) We divided the samples according to several characteristics sex (female or male), residence areas (urban or rural), education (educated or illiterate), and often exercise frequency (yes or not) and modelled separately to test the potential heterogeneity. (2) P for interaction indicates whether there was a significant difference across sex, residence areas, education and exercise frequency. (3) We utilised the STATA command stptime to calculate incidence rates per 1000 person-years. (4) All models were multivariable-adjusted for age (years), sex (male or female), illiterate (yes or no), marriage (in marriage or not), ethnic group (Han or minority), urban/rural residence, geographic region by representative taste preference (salty areas, sweet areas or hot areas), regular exercise (yes or no), smoke now (yes or no), often drink alcohol (yes or no), overweight (yes or no), self-rated health status (bad, so so and good), ADL (independent, mild dependent, moderate dependent and very dependent), and cardiometabolic diseases including hypertension (yes or no), diabetes (yes or no), heart disease (yes or no), cerebrovascular disease (yes or no), and dyslipidemia (yes or no). (5) ADL, activities of daily living; HR, hazard ratios.
Among older adults without CMD, a similar association between taste preference and MCI was observed (Fig. 4, Panel A). However, as the number of CMD increased, the significance of taste preference diminished. For older adults with a single CMD, only sweet taste preference had a significant detrimental effect (2·34, 95 % CI (1·82, 3·01)). Moreover, for older adults with two or more coexisting CMD, taste preference no longer had a significant effect. Nonetheless, sweet-liking individuals in this group still had the highest incidence rate of MCI (66·67 per 1000 person-years).
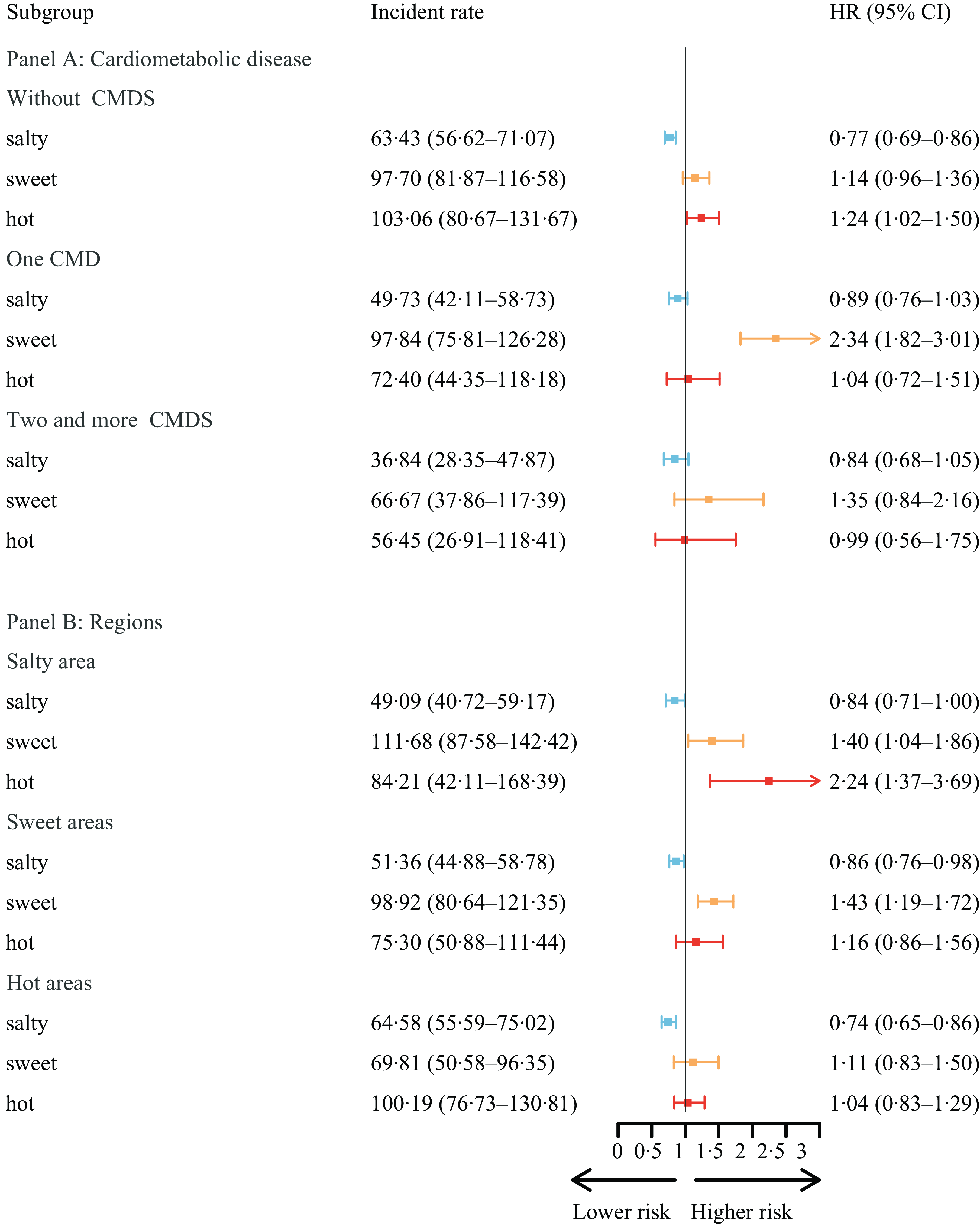
Fig. 4. The association between MCI and taste, by CMD and regional taste. (1) We divided the samples according to CMD and geographical regions by taste preference and modelled separately to test the potential heterogeneity. (2) Pfor interaction indicates whether there was a significant difference across CMD and geographical regions by representative taste preference. (3) We utilised the STATA command stptime to calculate incidence rates per 1000 person-years. (4) Panel A model was adjusted for age (years), sex (male or female), illiterate (yes or no), marriage (in marriage or not), ethnic group (Han or minority), urban/rural residence, geographic region by representative taste preference (salty areas, sweet areas or hot areas), regular exercise (yes or no), smoke now (yes or no), often drink alcohol (yes or no), overweight (yes or no), self-rated health status (bad, so so and good) and ADL (independent, mild dependent, moderate dependent and very dependent). (5) Panel B model was adjusted for age (years), sex (male or female), illiterate (yes or no), marriage (in marriage or not), ethnic group (Han or minority), urban/rural residence, regular exercise (yes or no), smoke now (yes or no), often drink alcohol (yes or no), overweight (yes or no), self-rated health status (bad, so so and good), ADL (independent, mild dependent, moderate dependent, very dependent), and CMD including hypertension (yes or no), diabetes (yes or no), heart disease (yes or no), cerebrovascular disease (yes or no), and dyslipidemia (yes or no). (6) MCI, mild cognitive impairment; CMD, cardiometabolic diseases; ADL, activities of daily living; HR, hazard ratios.
Considering the heterogeneity across geographical regions by taste preference (Fig. 4, Panel B), compared with people with insipidity taste, the salty taste was always the protective factor across the three regions. In salty regions, the risk of the participants developing poor cognitive function with hot preference increased by 124 % (2·24, 95 % CI (1·37, 3·69)) compared with that of people with insipid taste. As expected, for participants living in sweet regions, the detrimental effect of sweet taste was more prominent (1·43, 95 % CI (1·19, 1·72)). However, in hot areas, the effect of hot was no longer significant.
Discussion
The associations between dietary patterns and cognitive function have been extensively described in a growing body of literature, with many studies demonstrating that adherence to the plant diary was associated with better cognitive function(Reference Zhu, Chen and Shen6,Reference Lourida, Soni and Thompson-Coon7,Reference Psaltopoulou, Sergentanis and Panagiotakos8,Reference Boumenna, Scott and Lee9,Reference Morris, Tangney and Wang10) . The strengths of the study include the availability of data from a large cohort of older adults with 10 years of follow-up and accessibility to a wide range of measures, including baseline demographic, health and dietary patterns. This study strengthens and extends the existing evidence about the relationship between diet and cognitive function by examining the role of taste in the risk of developing MCI. Furthermore, our findings indicate that subjective taste preferences have a substantial impact on cognitive function, even when objective dietary patterns related to salt-preserved vegetables, sugar and garlic are considered. This provides a more familiar and user-friendly dietary guide for older adults, who may have relatively limited knowledge of nutritional aspects, making it more practical and suitable for implementation in their daily lives.
Specifically, this study makes several important advances in the knowledge pool of how taste preference could affect cognitive impairment. First, we demonstrated that sweet-liking or hot-liking people were more likely to develop MCI. However, salty taste was a potential protective factor for cognitive function, and the association remained significant in the adjusted model and subgroups. Second, the link was more evident for sweet-liking urban residents and hot-liking illiterate participants. Notably, cognitive decline related to sweet taste was prominent in participants with CMD. Among sweet-liking individuals, those with one CMD experienced a significant detrimental effect and those with co-occurring CMD had a higher incidence rate of MCI. Furthermore, the detrimental effect of sweet taste was intensified for people who lived in sweet areas.
However, there is no specific research available regarding the mechanisms by which sweetness could increase the risk of cognitive dysfunction. There is substantial and consistent evidence that sweetness has increased the risk of negative health outcomes, including obesity, type 2 diabetes and CVD(Reference Imamura, O’Connor and Ye37,Reference Malik, Li and Pan38) . Obesity, diabetes and related disorders may affect cognitive function through mechanisms including leptin’s influence on β-amyloid levels, inflammatory responses, adiponectin’s blood-brain barrier moderation, insulin resistance, microvascular disease and hormonal influences on cognition(Reference Profenno, Porsteinsson and Faraone24). One animal model showed that the chronic consumption of sweetness can change brain neurochemistry(Reference Hamelin, Poizat and Florian39). One case-control study found that spicy food consumption showed close inverse associations with cognition level(Reference Tian, Wang and Sun40), and one longitudinal study also proved the detrimental effect of chili intake(Reference Shi, El-Obeid and Riley41). Supporting these findings, we found that sweet (1·39, 95 % CI (1·22, 1·59)) and hot tastes (1·20, 95 % CI (1·02, 1·42)) showed strong evidence of deleterious effects on cognitive function.
However, inconsistent with previous studies, people with salt-like taste may be less likely to develop poor cognitive function for several reasons. Prior research has demonstrated that excessive salt intake plays a role in tau-related cognitive decline and may increase the risk of cognitive impairment(Reference Faraco, Hochrainer and Segarra42,Reference Mohan, Yap and Reidpath43) . However, one study employed a food frequency questionnaire to gauge dietary Na intake and demonstrated that Na intake does not impact cognitive function(Reference Nowak, Fried and Jovanovich44), and a cross-sectional study suggested that lower dietary Na was associated with poorer cognitive performance(Reference Rush, Kritz-Silverstein and Laughlin45). A possible explanation for the salt’s protective effect could stem from sample selection. Our study encompasses older adults aged 65 to 90 years, with a mean baseline age of 78·21 years (sd = 7·65). These relatively long-lived older adults, due to survival bias, are likely to be healthier and therefore more resilient to the adverse effects of high-salt diets. Additionally, considering the generally high salt intake in Chinese diets and the recent advocacy for lower-salt diets (such as the ‘Three Reductions’ recommended by the Chinese CDC), individuals who self-report a high-salt diet may be more conscious of their salt intake. Conversely, some individuals who self-report a ‘low-salt preference’ may actually have a high-salt diet but may not be aware of it. Consequently, some of the ‘high-salt’ diet group may be more vigilant in monitoring their salt intake, conferring a protective effect. As previously noted, MCI was found to have an adverse impact on the ability to discern salty tastes(Reference Cecchini, Federico and Zanini14). Consequently, individuals with compromised cognitive function may struggle to accurately perceive salty flavours, potentially leading them to label their taste preferences as insipid. Although the association between salty taste preference and MCI remains unclear, our results suggest that, concerning MCI prevention, old adults who reported an insipidity taste preference rather than salty taste cannot be ignored either.
The negative relationship between sweet-liking and cognitive behaviour was more pronounced in urban residents (1·46, 95 % CI (1·21, 1·76)), which might be explained by the long-term consumption of artificial sweeteners, which could impair cognitive functions(Reference Hamelin, Poizat and Florian39). Therefore, we should pay more attention to the prevention of MCI in urban older adults with a sweet tooth, especially since such people are likely to be the majority of urban older adults. As it is well known that education is a confounding variable for cognitive function, a previous study found that for people with different educational levels, the role of chili intake in cognitive function was significantly different(Reference Shi, El-Obeid and Riley41). Our study also showed an intensified deleterious effect for hot-liking participants without formal education (1·81, 95 % CI (1·44, 2·27)), whereas the association was statistically insignificant for educated people. Previous studies have shown that hot taste preference may be more popular among physical labour workers with low education because of the cooling effect from sweating due to eating spicy food(Reference Nabhan46). Another major reason might be the limitation of the health literature, which made it difficult for them to adhere to a healthy diet to mitigate the adverse effect of overconsumption of spicy food. Therefore, illiterate people also deserve more attention.
Although taste preference may drive food choice and dietary intake and further impact the risk of CMD, especially for those who prefer fatty and sweet foods(Reference Lampuré, Adriouch and Castetbon47), our studies revealed that the association between taste preference and MCI remains stable among older adults without CMD. However, among sweet-liking individuals, only those with one CMD experienced a significant detrimental effect, while all of them exhibited the highest incidence rate of MCI. Considering that older adults with one or more coexisting CMD have a significantly higher chance of developing MCI(Reference Kesse-Guyot, Julia and Andreeva30), the role of taste preference may be weakened. We emphasise that among older adults with CMD, even if they pay greater attention to their dietary habits (which may exacerbate their sensory liking), a higher preference for sweet foods remains a risk factor for cognitive function decline and warrants further attention.
Taste preference is closely associated with geographic regions and may further influence the taste threshold(Reference Baharuddin and Sharifudin31). Consistently, our results also supported the moderating effect of geographical regions. It was interesting that the hot taste was no longer a significant deleterious risk factor for MCI in regions with hot taste preference, while the sweet taste remained significant in sweet areas. One mechanism that may account for variations in different geographical regions is that people in areas with spicy food taste preferences may have a higher taste detection threshold(Reference Trachootham, Satoh-Kuriwada and Lam-Ubol48), leading to a higher tolerance. An additional possibility for varied effects is the divergent effects of sweet and hot taste preferences on our health. A spicy diet might be protective against CVD and metabolic syndrome disease(Reference Nilius and Appendino49), while the detrimental effects of sweetness have been well elucidated.
The WHO suggests that individuals consume more fruit and vegetables and recommends a healthy, balanced diet to reduce the risk of cognitive decline(50). Avoiding a sweety or spicy diet may also play a central role in cognitive function protection, and the illiterate group was especially the major concern.
There were some limitations in our study. Taste preference is the result of a complex interplay of environmental and socio-economic influences. Because the survey only recorded the status of older people after the age of 65 years, the effect of taste during the subjects’ lifespan is unknown, and the underlying mechanisms should be considered in future research. In addition, taste preference was self-reported, which may not be objective enough. However, it also has a certain rationality because self-reported indicators have been widely used in many areas, especially in the field of health research, that is, self-reported cognitive measures to predict the future risk of cognitive decline(Reference Wion, Hill and Bell51). Additionally, the MMSE is primarily employed as a screening tool for detecting potential cognitive impairment, often necessitating subsequent evaluation by experts for a formal diagnosis of cognitive disorders. Nevertheless, MMSE has demonstrated both accuracy and clinical utility, particularly when applied without expert supervision in community and primary care settings(Reference Mitchell20). We also employed various cut-off points tailored to individuals’ educational backgrounds to enhance its diagnostic accuracy. It is worth noting that the population with CMD may take medications, which could potentially have an impact on cognition. However, this aspect of medication use was not taken into account in the study and will be further addressed in future research. Finally, considering that there are multiple connections between taste preference, food choice and excess food consumption(Reference Drewnowski52), the people who reported having a salty taste do not necessarily intake more salty food than people reported to have an insipid taste. As a result, our analyses could have an objective quantitative bias to taste preference. Combined with the quantitative evidence from other types of studies, this study will increase the credibility of the results.
Conclusions
To summarise, taste, beyond its enjoyable aspect, plays an essential role in the cognitive function of older adults. Data evidence consistently shows that the hot and sweet taste was the risk factor and that the effect may be moderated by regional taste preference. The significance of hot taste as a risk factor for MCI diminishes in regions where it is commonly preferred, while the sweet taste remains significant in sweet areas. The sweet-liking urban residents and hot-liking illiterate participants were high-risk people. However, a preference for salty taste may be protective against cognitive impairment, and its underlying mechanism needs to be further explored. Promoting a harmonious and balanced taste among older adults is crucial for protecting their cognitive function and overall health. Targeted strategies should prioritise assisting individuals with CMD in reducing excessive intake of sweet foods while concurrently receiving appropriate treatment for CMD to safeguard cognitive function.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number 82103955), Clinical Medicine Plus X – Young Scholars Project, Peking University, the Fundamental Research Funds for the Central Universities (grant number 7100604313), and the Fundamental Research Funds for the Central Universities (grant number 710150319).
D. Y. wrote the first draft of the manuscript. D. Y., H. T. and C. G. were involved in data cleaning and analysis. C. G. formulated the research questions and designed the study. D. Y., H. T., P. Y. and C. G. contributed to the interpretation of the results and critical revision of the manuscript. All authors have read and approved the final version of the manuscript. C. G. is the study guarantor.
The authors declare no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523002593







