Ageing is associated with bone loss, and postmenopausal women are at a particularly high risk of osteoporosis, as oestrogen deficiency is the other primary factor in increasing bone resorption( Reference Khosla, Melton and Riggs 1 ). Oestrogen deficiency has been known to up-regulate osteoclast formation, which is mainly induced by the increased production of bone-resorbing inflammatory cytokines such as IL-1β, IL-6, IL-7 and TNF-α ( Reference Sipos, Pietschmann and Rauner 2 ). In ovariectomised (OVX) rats, 17β-oestradiol-3-benzoate (E2) treatment maintained bone volume (BV) by regulating both bone resorption and bone formation( Reference Chow, Tobias and Colston 3 ). In in vitro studies, E2 exerted anti-resorptive efficacy by regulating the receptor activator of NF-κB ligand (RANKL), an important determinant of osteoclast differentiation, and osteoprotegerin (OPG), which binds to and inactivates RANKL( Reference Bord, Ireland and Beavan 4 – Reference Hofbauer, Khosla and Dunstan 6 ). In addition, E2 increased the activity of runt-related transcription factor 2 (RUNX2)( Reference McCarthy, Chang and Liu 7 ), an essential factor for differentiation of osteoblasts.
In addition to oestrogen, the n-3 PUFA EPA (20 : 5n-3) and DHA (22 : 6n-3) are known to reduce osteoclastic bone resorption by decreasing the production of eicosanoids and inflammatory cytokines( Reference Kajarabille, Diaz-Castro and Hijano 8 ). In Fat-1 OVX mice, a transgenic model synthesising n-3 PUFA from n-6 PUFA, osteoblastogenesis was promoted by the inhibited expression of PPAR-γ and the enhanced expression of RUNX2 in bone marrow, which resulted in increased bone mass compared with wild-type OVX mice( Reference Chen, Zhang and Zheng 9 ).
There have been several studies investigating the combined effects of n-3 PUFA and E2 treatment on bone mass and architecture( Reference Schlemmer, Coetzer and Claassen 10 – Reference Sacco, Jiang and Reza-Lopez 12 ). A combination of E2 treatment and dietary supplementation of γ-linolenic acid+EPA( Reference Schlemmer, Coetzer and Claassen 10 ), DHA( Reference Poulsen, Moughan and Kruger 11 ) or flaxseed oil rich in α-linolenic acid( Reference Sacco, Jiang and Reza-Lopez 12 ) was more effective in preserving OVX-induced bone loss compared with either treatment alone. However, there have been no studies examining the underlying mechanisms by which n-3 PUFA alone or n-3 PUFA with E2 enhanced bone-protective efficacy. Therefore, the purpose of the present study was to investigate the synergistic bone-protective mechanism of the combined treatment of EPA+DHA supplementation and E2 injection in OVX rats.
Methods
Animals and diet
This animal protocol was approved by the Institutional Animal Care and Use Committee of Hanyang University (HY-IACUC-12-076). In total, 3-week-old female Wistar rats (Jung Ang Lab. Animal Inc.) were kept in ventilated cages in an air-conditioned room under controlled temperature (22±1°C) and humidity (40–50 %) with a 12-h light–12 h dark cycle. Rats had free access to food pellets and tap water. Rats were weighed once a week, and food intake was measured every day. A modified American Institute of Nutrition-93G diet with 10 % fat relative to total energy intake was used. The diet contained 42·94 g/kg of fish oil (Cenovis Health Company) and/or grape seed oil (CJ Cheiljedang). Grape seed oil was used instead of soyabean oil, which contained 18 : 3n-3. The diets contained 0 %, 1 % or 2 % of EPA+DHA relative to the total energy and contained 0, 8·09 and 16·21 g of fish oil, 0·2, 5·3 and 10·4 g of n-3 PUFA, and 31·4, 26·2 and 20·9 g of n-6 PUFA per kg diet, respectively. The fatty acid composition of the diet is shown in Table 1, as previously described in detail( Reference Oh, Jin and Park 13 ). In brief, the 0 %, 1 % and 2 % EPA+DHA diets contained 0·44, 0·47 and 0·49 % of 18 : 3n-3, 0·02, 6·17 and 12·34 of 20 : 5n-3, 0·02, 0·86 and 1·71 % of 22 : 5n-3, and 0·01, 4·86 and 9·74 % of 22 : 6n-3, respectively.
Table 1 Fatty acid composition of the diets
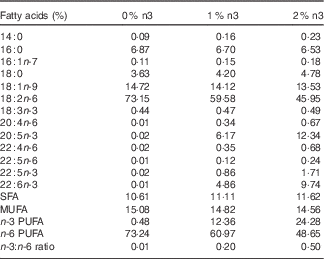
0 %, 1 % and 2 % n3, % n-3 PUFA relative to the total energy intake in the diet.
Experimental design
After a 1-week acclimatisation, 60 rats (4 weeks old) were randomly divided into one of three isoenergetic experimental diets (0 %, 1 % and 2 % EPA+DHA) for 12 weeks (n 20 per diet). At 8 weeks, rats (11 weeks old) were surgically OVX under anaesthesia. After a 1-week recovery period from ovariectomy, rats (n 10 per group) were randomly assigned to E2- or maize oil-injected groups (Sigma-Aldrich): 0 % EPA+DHA diet with maize oil injection (0 % n3), 0 % EPA+DHA diet with E2 injection (0 % n3+E2), 1 % EPA+DHA diet with maize oil injection (1 % n3), 1 % EPA+DHA diet with E2 injection (1 % n3+E2), 2 % EPA+DHA diet with maize oil injection (2 % n3) and 2 % EPA+DHA diet with E2 injection (2 % n3+E2). The schematic diagram for group allocation is presented in Fig. 1. To mimic the rat oestrous cycle, 1 ml of maize oil with or without 10 μg of E2 was subcutaneously injected every 4 days during the last 3 weeks of the experiment, with the final administration performed 24 h before killing. Rats (15 weeks old) were fasted overnight and were anaesthetised with zoletil (25 mg/kg) and rompun (10 mg/kg). Blood samples were collected in serum-separating tubes and EDTA blood tubes, and serum and plasma were obtained after centrifugation. Tissues were collected, rinsed with saline and weighed at the time of killing. Femura and tibiae were dissected, and the length and weight of the bones were measured. Blood and tissue samples were snap-frozen in liquid N2 and stored at −80°C for further analysis.

Fig. 1 Schematic diagram of group allocation. After a 1-week acclimatisation period, sixty rats were randomly divided into one of three isoenergetic experimental diets (0 %, 1 % and 2 % EPA+DHA) for 12 weeks (n 20 per group). At 8 weeks, rats were surgically ovariectomised, and after a 1-week recovery period from ovariectomy, rats were randomly assigned to 17β-oestradiol-3-benzoate (E2)- or maize oil-injected groups (n 10 per group). 0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with E2 injection.
Measurement of blood levels of 17β-oestradiol-3-benzoate and eicosanoids
Plasma level of E2 (Cayman Chemical Company) and serum levels of PG E2 (Cayman Chemical Company) and leukotriene B4 (LTB4; R&D Systems) were measured using ELISA according to the manufacturer’s instructions. All measurements were quantified relative to standards in a spectrophotometer (Multiscan GO; Thermo Scientific).
Determination of blood levels of calcium, phosphate and bone turnover markers
Serum levels of calcium (Ca) and phosphate (P) were measured using colorimetric methods (BioAssay Systems) and analysed using a microplate reader (iMark Microplate Reader; Bio-Rad). Serum levels of bone formation markers such as osteocalcin (OC; Nordic Bioscience) and bone-specific alkaline phosphatase (BSALP; MyBioSource) and a bone resorption marker, C-terminal cross-linked telopeptides of type I collagen (CTX; Nordic Bioscience), were measured using ELISA according to the manufacturer’s instructions. The results were quantified in a spectrophotometer.
GC
Total lipids were extracted from powdered femur (n 10 per group; 300 mg) by homogenisation in 5-ml chloroform–methanol–distilled water (2:2:1, v/v), and phospholipids were separated with TLC (Silica gel G; Analtech) using hexane–diethyl ether–acetic acid (40:10:1, v/v). Phospholipids were methylated by adding boron trifluoride methanol–benzene (B1252; Sigma-Aldrich), and were heated at 100°C for 10 min. Fatty acid methyl esters were analysed using GC (Shimadzu 2010AF; Shimadzu Scientific Instruments). An external standard (GLC-727; Nu-Check Prep) was used for identification of fatty acids, and the CV was 2·0 %.
Micro-computed tomography
Left femora (n 5 per group) were fixed in 4 % paraformaldehyde after killing. A high-resolution micro-computed tomography system (InspeXio SMX-90CT, Shimadzu, Kyoto, Japan) was used to scan the proximal region of the femora. The micro-computed tomography (μCT) machine is equipped with a 90-kV scanning voltage, 10-W power, 109-μA current and 9-μm scan thickness. The femora were placed on a holder between the X-ray source and the flat panel detector (inspeXio SMX-90CT; Shimadzu). The scanning angular rotation was 360°, and the angular increment was 1·6°. After scanning the data, reconstruction of three-dimensional data sets with a voxel size of 9 μm was conducted. Both femoral trabecular and cortical bones were analysed using the commercial software provided with the equipment (TRI3D-BON Analysis software; RATOC).
Western blot analysis
Frozen femora (n 10 per group) were ground into a fine powder with a mortar and pestle under liquid N2. Bone powder was homogenised in an ice-cold radioimmunoprecipitation buffer containing complete EDTA-free protease and PhosSTOP inhibitor cocktails (Roche Diagnostics GmbH). The homogenates were centrifuged at 10 000 g for 15 min at 4°C, and the protein concentration of the supernatant was determined using a bicinchoninic assay (Pierce Biotechnology). Proteins (30 μg) were separated on 8–10 % SDS-PAGE, transferred to polyvinylidene fluoride membranes and blocked for 1 h at room temperature with 5 % skimmed milk or 5 % bovine serum albumin in Tris-buffered saline containing Tween 20 (TBST). The membrane was then incubated with anti-PPAR-γ (1:400), RUNX2 (1:500), OPG (1:1000), RANKL (1:2000), TNF-α (1:1000), IL-6 (1:1000), IL-1β (1:1000), IL-7 (1:400) and oestrogen receptor-α (ER-α; 1:500) or ER-β (1:1000) with 5 % skimmed milk in TBST overnight at 4°C. All antibodies were purchased from Abcam, except for anti-IL-7. After rinsing in TBST three times for 5 min each, membranes were incubated with horseradish-peroxidase-conjugated secondary antibody, which was either anti-rabbit IgG (Cell signaling, New England Biolabs) or anti-mouse IgG (Enzo Life Science) with 5 % skimmed milk in TBST for 1 h at room temperature. Immunoreactive bands were visualised on the UV setting using the ChemiDoc MP Imaging System (Bio-Rad) to estimate total protein per lane, and β-actin was used for normalisation.
Statistical analysis
Values are expressed as means with their standard errors , and differences were considered significant at P<0·05. Data were analysed using a two-way ANOVA with factors of n-3 PUFA diet and E2 injection, followed by Duncan’s post hoc test. Analyses were conducted using SPSS for Windows, version 18.0 (SPSS Inc.).
Results
Dietary intake, body weight and organ weights
There were no significant differences in initial body weight, weights of the liver or kidneys, and weight and length of the tibia and femur (Table 2). Regardless of n-3 PUFA supplementation, E2-injected rats consumed less food and had a lower final body weight but heavier uterus weight relative to maize oil-injected rats.
Table 2 Dietary intake and body and organ weights (Mean values with their standard errors; n 10 per group)

0 %, 1 % and 2 % n3, 0 % 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with 17β-oestradiol-3-benzoate injection.
* Mean values was significantly different between maize oil and E2 injection within the diets containing the same amount of n-3 PUFA at P<0·05.
Blood concentrations of 17β-oestradiol-3-benzoate, eicosanoids, calcium, phosphate and bone turnover markers
E2 injection significantly increased the plasma concentration of E2 and the serum level of Ca but decreased the serum levels of OC, BSALP and CTX regardless of n-3 PUFA supplementation (Table 3). Both n-3 PUFA supplementation and E2 injection decreased the serum level of LTB4, and an additive inhibitory effect was observed in the combined treatment of n-3 PUFA and E2. There were no effects of n-3 PUFA or E2 on the blood levels of PG E2 and P.
Table 3 Blood levels of 17β-oestradiol-3-benzoate (E2), eicosanoids, calcium, phosphate and bone turnover marker levels (Mean values with their standard errors; n 10 per group)

0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2; 0 %, 1 % and 2 % EPA+DHA diet with 17β-oestradiol-3-benzoate injection; LTB4, leukotriene B4; OC, osteocalcin; BSALP, bone-specific alkaline phosphatase; CTX, C-terminal cross-linked telopeptides of type I collagen.
* Values are significantly different between maize oil and E2 injection within the diets containing the same amount of EPA+DHA at P<0·05.
** Values are significantly different among 0 %, 1 % and 2 % n3 within the maize oil- or E2-injected group at P<0·05.
Fatty acid composition of femur phospholipids
n-3 PUFA supplementation significantly increased the bone phospholipid levels of 18 : 2n-6 and n-3 PUFA such as 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3, whereas supplementation decreased the levels of n-6 PUFA such as 20 : 4n-6, 22 : 4n-6 and 22 : 5n-6 in a dose-dependent manner (Table 4). E2 injection significantly increased the bone phospholipid level of 18 : 0 but decreased the levels of 18 : 1n-9 and MUFA.
Table 4 Fatty acid compositions of femur phospholipids (Mean values with their standard errors; n 10 per group)

0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with 17β-oestradiol-3-benzoate injection.
* Values are significantly different between maize oil and E2 injection within the diets containing the same amount of EPA+DHA at P<0·05.
** Values are significantly different among 0 %, 1 % and 2 % n3 within maize oil- or E2-injected groups at P<0·05.
*** Values are significantly different between 1 % and 2 % n3 within maize oil- or E2-injected groups at P<0·05.
The expressions of cytokines, osteoprotegerin, receptor activator of NF-κB ligand, PPAR-γ and runt-related transcription factor 2 in bone
E2 injection significantly decreased the bone expression of the inflammatory cytokines IL-1β, IL-6, IL-7 and TNF-α regardless of n-3 PUFA supplementation (Fig. 2). n-3 PUFA supplementation significantly decreased the bone expressions of IL-7 and TNF-α regardless of E2 injection, but decreased the bone expressions of IL-1β and IL-6 only in rats injected with E2. In addition, there were significant interactions between n-3 PUFA and E2 in the bone expression of IL-1β (P=0·021), indicating the synergistic reduction of IL-1β expression by the combination of n-3 PUFA and E2.

Fig. 2 Effects of n-3 PUFA supplementation and 17β-oestradiol-3-benzoate (E2) on the bone expressions of (a) IL-1β, (b) IL-6, (c) IL-7, (d) TNF-α and (e) representative images of Western blotting. Values are means (n 10 per group), with their standard errors are represented by vertical bars. 0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with E2 injection. * Values are significantly different between maize oil and E2 injection within the diets containing the same amount of EPA+DHA at P<0·05. ** Values are significantly different among 0 %, 1 % and 2 % n3 within maize oil- or E2-injected groups, at P<0·05. NC, 0 % n3.
E2 injection, but not n-3 PUFA supplementation, significantly increased the bone expression of OPG and decreased the bone expression of RANKL (Fig. 3). Both n-3 PUFA supplementation and E2 injection decreased the bone expression of PPAR-γ. The bone expression of RUNX2 was increased by E2 injection only in rats supplemented with n-3 PUFA and by n-3 PUFA supplementation only in rats injected with E2. Interactions between n-3 PUFA and E2 were also seen in the bone expression of RUNX2 (P=0·041), suggesting a synergistic increase in RUNX2 expression by the combination of n-3 PUFA and E2.

Fig. 3 Effects of n-3 PUFA supplementation and 17β-oestradiol-3-benzoate (E2) on the bone expressions of (a) osteoprotegerin (OPG), (b) receptor activator of NF-κB ligand (RANKL), (c) PPAR-γ, (d) runt-related transcription factor 2 (RUNX2) and (e) representative images of Western blotting. Values are means (n 10 per group), with their standard errors are represented by vertical bars. 0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with E2 injection. * Values are significantly different between maize oil and E2 injection within the diets containing the same amount of EPA+DHA at P<0·05. ** Values are significantly different among 0 %, 1 % and 2 % n3 within maize oil- or E2-injected groups, at P<0·05. NC, 0 % n3.
Micro-computed tomography analysis (bone microarchitecture)
Representative μCT images of the analysed region of the femur are shown in Fig. 4. There was no significant difference in femoral trabecular bone mineral density (BMD) after either n-3 PUFA supplementation or E2 injection. Femoral trabecular BV/tissue volume (TV) and bone mineral content (BMC)/TV were significantly increased by E2 injection regardless of n-3 PUFA supplementation, but were increased by n-3 PUFA supplementation only in rats with E2 injection. There was a marginally significant interaction between n-3 PUFA supplementation and E2 injection on the femoral trabecular BV/TV (P=0·056).

Fig. 4 Micro-computed tomography analysis of the proximal femur from control and n-3 PUFA-supplemented and/or 17β-oestradiol-3-benzoate (E2)-injected rats. (a) Representative three-dimensional images of the proximal femur. Effects of n-3 PUFA and E2 on femoral trabecular (b) bone mineral density (BMD), (c) bone volume (BV)/tissue volume (TV) and (d) bone mass content (BMC)/TV. Effects of n-3 PUFA and E2 on femoral cortical (e) BMD, (f) BV and (g) BMC. Values are means (n 10 per group), with their standard errors are represented by vertical bars. 0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with E2 injection. * Values are significantly different between maize oil and E2 injection within the diets containing the same amount of EPA+DHA at P<0·05. ** Values are significantly different among 0 %, 1 %, and 2 % n3 within maize oil- or E2-injected groups, at P<0·05.
There was no significant difference in femoral cortical BMD or BV caused by either n-3 PUFA supplementation or E2 injection. However, femoral cortical BMC was increased by n-3 PUFA supplementation only in rats with E2 injection. There was a significant interaction between n-3 PUFA supplementation and E2 injection on femoral cortical BV (P=0·008) and BMC (P=0·034), suggesting synergistic increases in cortical BV and BMC by the combination of n-3 PUFA and E2.
The expressions of oestrogen receptor-α and oestrogen receptor-β in bone
E2 injection and n-3 PUFA supplementation significantly increased the bone expression of ER-α (Fig. 5). However, there was no significant effect of E2 injection or n-3 PUFA supplementation on the bone expression of ER-β.

Fig. 5 Effects of n-3 PUFA supplementation and 17β-oestradiol-3-benzoate (E2) on the bone expressions of (a) oestrogen receptor-α (ER-α), (b) ER-β and (c) representative images of Western blotting. Values are mean (n 10 per group), with their standard errors are represented by vertical bars. 0 %, 1 % and 2 % n3, 0 %, 1 % and 2 % EPA+DHA diet with maize oil injection; 0 %, 1 % and 2 % n3+E2, 0 %, 1 % and 2 % EPA+DHA diet with E2 injection. * Values are significantly different between maize oil and E2 injection within the diets containing the same amount of EPA+DHA at P<0·05. ** Values are significantly different among 0 %, 1 % and 2 % n3 within maize oil- or E2-injected groups, at P<0·05.
Discussion
This was the first study to demonstrate that the synergistic bone-protective efficacy of n-3 PUFA and E2 was induced by suppression of bone-resorbing cytokine IL-1β, followed by the enhanced bone expression of RUNX2, the major transcription factor in osteoblast differentiation.
A combination of E2 treatment and dietary supplementation of n-3 PUFA, including γ-linolenic acid+EPA( Reference Schlemmer, Coetzer and Claassen 10 ), DHA( Reference Poulsen, Moughan and Kruger 11 ) or flaxseed oil rich in α-linolenic acid( Reference Sacco, Jiang and Reza-Lopez 12 ), has been reported to be more effective in preserving OVX-induced bone loss than if used separately. Consistent with previous studies, the present study found a synergistic combined effect of n-3 PUFA (EPA+DHA) and E2 on femoral cortical BMC and BV, as well as on bone expressions of RUNX2 and IL-1β. RUNX2 is well known for its vital role in osteoblastogenesis( Reference Vimalraj, Arumugam and Miranda 14 ), whereas IL-1β is one of the major cytokines stimulating osteoclast differentiation and accelerating bone resorption( Reference Pacifici 15 ). Ding et al.( Reference Ding, Ghali and Lencel 16 ) reported that RUNX2 expression is inhibited by IL-1β in human mesenchymal stem cells (MSC), suggesting an interaction between IL-1β and RUNX2. A synergistic decrease in IL-1β expression through the combined treatment of n-3 PUFA and E2 might result in a subsequent up-regulation of RUNX2, which can contribute to the maintenance of bone mass and BV observed in the present study. On the other hand, RUNX2 has been reported to be associated with the bone-specific signalling of E2 through an ER-α dependent pathway( Reference McCarthy, Chang and Liu 7 ). As the present study showed that the combined use of n-3 PUFA and E2 enhanced the ER-α expression, RUNX2 expression might be modulated through an ER-α-dependent pathway.
Consistent with previous studies( Reference Chow, Tobias and Colston 3 , Reference Lane, Haupt and Kimmel 17 ), the present study found that E2 treatment reduces OVX-induced bone loss and serum levels of bone turnover markers such as OC, BSALP and CTX. It is well known that oestrogen deficiency increases bone turnover and induces rapid bone loss( Reference Sims, Morris and Moore 18 ). In addition, our study showed a significant increase in serum levels of Ca in rats injected with E2, which is in line with previous results reporting that E2 treatment increased intestinal Ca absorption( Reference Arjmandi, Hollis and Kalu 19 ).
One of the mechanisms by which E2 regulates bone metabolism could be related to the suppression of osteoclast differentiation( Reference Sipos, Pietschmann and Rauner 2 ). Previous studies have shown that E2 treatment reduced osteoclastogenesis through stimulation of OPG secretion but decreased RANKL and cytokine expressions( Reference Ho, Santora and Chen 5 , Reference Hofbauer, Khosla and Dunstan 6 , Reference Pacifici 20 ), consistent with the present study. In addition, E2 promotes osteoblastogenesis by enhancing RUNX2 activity( Reference McCarthy, Chang and Liu 7 ) or by increasing the number of cells expressing RUNX2( Reference Plant, Samuels and Perry 21 ), which inhibits bone marrow adipogenesis by reducing PPAR-γ expression( Reference Elbaz, Rivas and Duque 22 ). The present study also found that the bone expression of RUNX2 significantly increased but that of PPAR-γ decreased with E2 treatment.
Oestrogen can act on the skeleton through two receptors, ER-α and ER-β, both of which are present in bone, although ER-α appears to mediate most actions of oestrogen in bone( Reference Nakamura, Imai and Matsumoto 23 ). In the present study, E2 increased the bone expression of ER-α but not of ER-β, consistent with a previous finding showing increased ER-α mRNA in human bone marrow cells treated with E2 ( Reference Ramalho, Couttet and Baudoin 24 ). Activation of ER-α by E2 suppressed osteoclastogenesis( Reference Nakamura, Imai and Matsumoto 23 ) and stimulated OPG expression in osteoblasts( Reference Bord, Ireland and Beavan 4 ).
Previous studies have shown that n-3 PUFA supplementation reduced OVX-induced bone loss in rats( Reference Poulsen, Moughan and Kruger 11 , Reference Watkins, Li and Seifert 25 , Reference Watkins, Reinwald and Li 26 ), whereas the present study found that n-3 PUFA prevented femoral BV and BMC depletion in OVX rats with E2 but not in those without E2. Although rats were fed n-3 PUFA for 12 weeks in the present study, the lack of bone-protective efficacy of n-3 PUFA alone might be due to the relatively short feeding period after OVX compared with previous studies (4 weeks v. 12–18 weeks)( Reference Poulsen, Moughan and Kruger 11 , Reference Watkins, Li and Seifert 25 , Reference Watkins, Reinwald and Li 26 ). As bone loss occurred rapidly after OVX( Reference Khosla, Melton and Riggs 1 ), the feeding period of n-3 PUFA after OVX should be longer than 12 weeks. In addition, the present study and another study( Reference Fernandes, Bhattacharya and Sun 27 ) used fish oil containing more EPA than DHA, which failed to prevent OVX-induced bone loss, whereas fish oil with high DHA levels demonstrated a bone-protective effect( Reference Fernandes, Bhattacharya and Sun 27 , Reference Li, Reinwald and Seifert 28 ).
In the present study, n-3 PUFA supplementation significantly decreased the serum level of LTB4 and the bone expressions of inflammatory cytokines, which are strong promoters of bone resorption and osteoclastogenesis( Reference Maggio, Artoni and Lauretani 29 , Reference Garcia, Boyce and Gilles 30 ). Rahman et al.( Reference Rahman, Bhattacharya and Banu 31 ) reported that increased n-3 PUFA levels in bone marrow phospholipids in Fat-1 OVX mice were accompanied by reduced production of inflammatory cytokines in bone marrow cells. However, there was no significant effect of n-3 PUFA on the bone expression of RANKL or its decoy receptor OPG, which are also important determinants of osteoclastic differentiation and activation( Reference Ho, Santora and Chen 5 ). Previous in vitro and in vivo studies examining the effects of n-3 PUFA on the RANKL/OPG pathway reported mixed results( Reference Coetzee, Haag and Kruger 32 – Reference Lappe, Kunz and Bendik 34 ). Sacco et al.( Reference Sacco, Chen and Ganss 35 ) reported that short-term (2 weeks) periods of n-3 PUFA (flaxseed oil) supplementation resulted in no changes in the serum level of RANKL or in the OPG:RANKL ratio in OVX rats, but that E2 treatment did result in such changes. Therefore, the lack of regulatory effects of n-3 PUFA on the RANKL/OPG pathway might compensate for the effects of reduced inflammatory cytokines on osteoclastic bone loss.
Another mechanism involved in the regulation of bone metabolism by n-3 PUFA is the alteration of osteoblast differentiation( Reference Watkins, Li and Lippman 36 ). Bone formation is mediated by the differentiation of MSC into adipocytes or osteoblasts, which are regulated by the key transcription factors PPAR-γ and RUNX2, respectively( Reference Chen, Zhang and Zheng 9 ). In the present study, n-3 PUFA suppressed PPAR-γ expression and complementarily increased the expression of RUNX2 by reducing bone loss in the presence of E2. It has been reported that factors related to MSC differentiation may affect the activity of other regulators, indicating the presence of cross-talk between different signalling pathways( Reference Takada, Kouzmenko and Kato 37 ). Thus, simultaneous regulation of PPAR-γ and RUNX2 by n-3 PUFA in the presence of E2 may additively contribute to osteoblastogenesis and preserve bone loss.
In line with previous studies( Reference Watkins, Reinwald and Li 26 , Reference Watkins, Li and Lippman 36 ), the present study showed that n-3 PUFA supplementation increased bone phospholipid levels of long-chain n-3 PUFA dose-dependently. In addition, the present study reported that n-3 PUFA supplementation decreased bone phospholipid levels of n-6 PUFA, except 18 : 2n-6. The increased 18 : 2n-6 level was because of the fact that the n-3 PUFA diet used in the present study contained 18 : 2n-6, and long-chain n-6 PUFA were decreased because of the competition of EPA and DHA in membrane incorporation( Reference Schmitz and Ecker 38 ). The decreased bone phospholipid levels of n-6 PUFA, especially 20 : 4n-6, the substrate for PG E2 synthesis, could partly contribute to the anti-inflammatory effect( Reference Kajarabille, Diaz-Castro and Hijano 8 ). In addition, the present study showed that E2 injection increased the bone phospholipid levels of 18 : 0 but decreased the levels of 18 : 1n-9, which might be due to the regulatory effects of E2 on stearoyl-CoA desaturase-1 and elongase 6 enzymes( Reference Marks, Kitson and Stark 39 ).
There are several limitations to the present study. There were no sham controls, which made it impossible to analyse OVX-induced bone loss. However, using OVX rats as the animal model for postmenopausal osteoporosis is well-established. In addition, the present study measured bone loss only in the femur, which might be a better site for reflecting osteoporotic bone loss( Reference Singh, Nagrath and Maini 40 ), rather than other sites. Although we injected E2 every 4 d to mimic the rat’s oestrous cycle, our finding could be applicable to the clinical setting that n-3 PUFA might have a bone-protective effect in postmenopausal women, especially who receive hormone-replacement therapy.
Conclusions
This is the first study to reveal that n-3 PUFA and E2 exert synergistic bone-protective efficacy through the suppression of bone-resorbing cytokine IL-1β followed by up-regulation of RUNX2, an essential transcription factor of bone formation. The present study suggested a new evidence for the interaction between IL-1β and RUNX2 on bone-protective effect by the combination of n-3 PUFA and E2. However, further clinical studies are needed to investigate whether the bone-protective efficacy of n-3 PUFA is greater in premenopausal or postmenopausal women receiving hormone therapy than in postmenopausal women not receiving hormone therapy.
Acknowledgements
The present study was supported by a Korean Research Foundation grant funded by the Korean Government (grant no. NRF-2015R1D1A1A09060823).
The authors’ contributions are as follows: Y. J. and Y. P. wrote the first draft of the study. Y. J performed experiment and statistical analysis and drafted the manuscript. Y. P. contributed to interpretation of the results, editing and to the final text. M. L. reviewed the manuscript. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.












