The health benefits of carotenoid pigment were first elucidated in mammals more than 80 years ago(Reference Green and Mellanby1), and the following decades of research have helped us to raise our interest and understanding of the functions of carotenoids in animals such as mammals, birds and fishes. A large body of experimental evidence has revealed that carotenoids modulate the immune system. For example, β-carotene supplementation increased CD4+, natural killer and IL-2 receptor cells(Reference Watson, Prabhala and Plezia2) and the ratio of CD4+-to-CD8+ cells(Reference Murata, Tamai and Morinobu3) in human peripheral blood, and inhibited the growth of mammary tumours in mice(Reference Chew, Park and Wong4), suggesting that β-carotene might be useful in immune regulation. Lutein could blunt systemic indices of inflammatory response in chicks(Reference Koutsos, Lopez and Klasing5), change lymphocyte subsets and proliferation in dogs(Reference Kim, Chew and Wong6) and cats(Reference Kim, Chew and Wong7), and inhibit mammary tumour growth and enhance phytohaemagglutinin-induced lymphocyte proliferation in mice(Reference Chew, Wong and Wong8). Recent studies reported that proinflammatory mediators and cytokines could be regulated by lutein(Reference Jin, Ohgami and Shiratori9), β-carotene(Reference Pang, Wang and Weng10, Reference Katsuura, Imamura and Bando11), β-cryptoxanthin(Reference Katsuura, Imamura and Bando11), astaxanthin(Reference Yasui, Hosokawa and Mikami12, Reference Lee, Bai and Lee13) and lycopene(Reference Simone, Russo and Catalano14, Reference Palozza, Simone and Catalano15). Therefore, modulation of proinflammatory and anti-inflammatory cytokines and other immune mediators' expression may be an important and significant mechanism for the immunomodulatory effects of carotenoids.
The intestine has a highly specialised immune system and constitutes one of the largest immunological organs. The intestine not only permits absorption of nutrients, but also maintains the ability to respond appropriately to a diverse milieu of dietary and microbial antigenic components(Reference Wittig and Zeitz16, Reference Burkey, Skjolaas and Minton17). The intestinal mucosa is challenged by diet-derived pro-oxidants (including Fe, Cu, H2O2, haem, lipid peroxides, aldehydes and nitrite), mutagens, carcinogens and endogenously generated reactive oxygen species, leading to oxidative stress in the gastrointestinal tract(Reference Ames18, Reference Halliwell, Zhao and Whiteman19). Carotenoids do not seem to be as well absorbed as vitamins C and E(Reference Halliwell, Zhao and Whiteman19), and hence their concentrations could be much higher in the lumen of the gastrointestinal tract than are ever achieved in plasma or other body tissues, making a functional action in the gastrointestinal tract more likely(Reference Halliwell, Zhao and Whiteman19). Many of the functions of T cells in the gastrointestinal immune system are mediated by secreted cytokines(Reference Wittig and Zeitz16). Hence, investigating the effects of carotenoids on cytokine expression of the gut is meaningful for our understanding of how carotenoids work in the gut.
A maize–soyabean basal diet is used in a wide geographical area (such as the USA and China) and lutein is added to pigment skin and eggs to satisfy consumer acceptance, and so both lutein and zeaxanthin exist in the diet. In the present study, we used a rice–soyabean basal diet and applied lutein and zeaxanthin as additives not only because lutein and zeaxanthin are the main carotenoids of the chicken yolk, but also because they have little or no provitamin A activity. There have been few studies pertaining to carotenoids on proinflammatory and anti-inflammatory cytokines of hens and chicks, especially in the intestine. Therefore, our objectives were to investigate the effects of xanthophylls on proinflammatory and anti-inflammatory cytokine expression in the liver and small intestine (duodenum, jejunum and ileum) of hens and chicks.
Materials and methods
Institutional and national guidelines for the care and use of animals were followed and all experimental procedures involving animals were approved by the Committee of Animal Experiments of South China Agricultural University (approval ID 201004152). All efforts were made to minimise suffering.
Expt 1
To examine the effects of dietary xanthophylls (containing 40 % of lutein and 60 % of zeaxanthin, as we determined; Juyuan Biochemical Company Limited) on proinflammatory cytokine (IL-1β, IL-6, interferon (IFN)-γ and lipopolysaccharide-induced TNF-α factor (LITAF)) and anti-inflammatory cytokine (IL-4 and IL-10) expression of breeding hens, 432 hens at 34 weeks of age with similar weight and genetic background were randomly assigned to three treatments. Each treatment was replicated six times and there were twenty-four breeding hens per replicate. The hens were fed either a carotenoid-depleted diet supplemented with 0 mg/kg xanthophylls (as the control group; containing 0·05 mg/kg xanthophylls, as we determined) or a carotenoid-replete diet supplemented with 20 or 40 mg/kg xanthophylls (containing 20·07 and 30·94 mg/kg xanthophylls, as we determined, respectively). The diets were formulated according to Chinese Feeding Standard of Chicken (2004) and NRC (1994). Details of ingredient composition and calculated nutrient content of diets for hens are provided in Table 1. The experiment lasted for 35 d, and water and diet were provided ad libitum. Production performance (egg number, total egg weight, feed intake, broken eggs, qualified eggs and hen mortality) of each replicate was recorded daily. Blood of hens (two hens for each replicate) was sampled at 7, 14, 21, 28 and 35 d after xanthophyll supplementation to determine the carotenoid content in plasma according to Koutsos et al. (Reference Koutsos, Clifford and Calvert20), which indicated that serum carotenoids reach a new steady state after 21 d of xanthophyll supplementation (Fig. S1, supplementary material for this article can be found at http://www.journals.cambridge.org/bjn). From 29 to 35 d of the trial, 510 eggs (eighty-five eggs from each replicate) were collected from the control group or the 40 mg/kg xanthophyll group, and hatched artificially to determine the fertilisation rate, hatchability of fertilised eggs, chick birth weight and healthy chick rate. The eggs were collected from 29 d of the trial because yolk carotenoid concentration had reached a new steady state after about 3 weeks of supplementation in the hens' diet, as we (Fig. S2, supplementary material for this article can be found at http://www.journals.cambridge.org/bjn) and other researchers have determined(Reference Karadas, Pappas and Surai21, Reference Surai and Speake22). On the 35th day of the trial, two hens from each replicate (twelve hens for each treatment) were weighed and slaughtered. Liver, duodenum, jejunum and ileum samples were collected immediately after slaughter and flash-frozen in liquid N2 for the determination of proinflammatory and anti-inflammatory cytokines.
Table 1 Basal diet composition and calculated nutrient content for meat-type breeding hens* and chicks†, on an as-fed basis
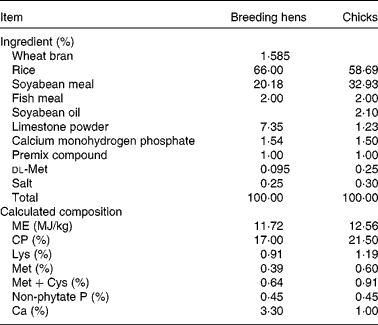
ME, metabolisable energy; CP, crude protein.
* Premix compound (mineral and vitamin mix) provided per kg of diet: vitamin A, 3·6 mg; cholecalciferol, 0·06 mg; vitamin E, 30 mg; menadione, 1·5 mg; riboflavin, 9·0 mg; niacin, 35 mg; d-pantothenic acid, 12 mg; vitamin B12, 0·012 mg; biotin, 0·2 mg; folacin, 1·2 mg; vitamin B1, 2·0 mg; vitamin B6, 4·5 mg; 8·0 mg of Cu from CuSO4·5H2O; 80 mg of Zn from ZnSO4·H2O; 100 mg of Mn from MnSO4·H2O; 80 mg of Fe from FeSO4·H2O; 1·0 mg of I from KI; 0·3 mg of Se from Na2SeO3.
† Premix compound (mineral and vitamin mix) provided per kg of diet: vitamin A, 2·4 mg; cholecalciferol, 0·025 mg; vitamin E, 20 mg; menadione, 0·5 mg; riboflavin, 8·0 mg; niacin, 35 mg; d-pantothenic acid, 10 mg; vitamin B12, 0·01 mg; biotin, 0·18 mg; folacin, 0·55 mg; vitamin B1, 2·0 mg; vitamin B6, 3·5 mg; 8·0 mg of Cu from CuSO4·5H2O; 100 mg of Zn from ZnSO4·H2O; 120 mg of Mn from MnSO4· H2O; 100 mg of Fe from FeSO4·H2O; 0·7 mg of I from KI; 0·3 mg of Se from Na2SeO3.
Expt 2
To examine the effects of in ovo and dietary xanthophylls (containing 40 % of lutein and 60 % of zeaxanthin) on proinflammatory cytokine (IL-1β, IL-6, IFN-γ and LITAF) and anti-inflammatory cytokine (IL-4 and IL-10) expression of chicks, a 2 × 2 factorial arrangement of treatments consisting of two in ovo xanthophyll levels and two dietary xanthophyll levels was designed. A total of 510 eggs were collected from hens fed a carotenoid-depleted diet or a 40 mg/kg xanthophyll diet in Expt 1. On the day of hatching, fertilisation rate, hatchability of fertilised eggs, chick birth weight and healthy chick rate were calculated, and 180 healthy male chicks from each in ovo xanthophyll treatment were chosen randomly and assigned to one of two dietary xanthophyll levels: a basal diet supplemented with 0 or 40 mg/kg xanthophylls (containing 0·07 and 40·02 mg/kg xanthophylls, as we determined, respectively). A total of twelve chicks (two chicks for each replicate) without feeding from each in ovo xanthophyll treatment were weighed and slaughtered within 12 h after hatching (0 d of chicks), following which the liver, duodenum, jejunum and ileum samples were removed immediately after slaughter and flash-frozen in liquid N2 for further analysis. There were four groups of progeny designed as follows: parents and chicks fed the carotenoid-replete diets, parents fed the carotenoid-replete and chicks fed the carotenoid-depleted diets, parents fed the carotenoid-depleted and chicks fed the carotenoid-replete diets, parents and chicks fed the carotenoid-depleted diets. Each of the four progeny groups contained six replicate pens with fifteen male chicks in each. The diets were formulated according to Chinese Feeding Standard of Chicken (2004) and NRC (1994). Details of ingredient composition and calculated nutrient content of diets for chicks are provided in Table 1. Dietary xanthophyll levels were chosen to be similar to those fed to commercial poultry. Chicks were housed in battery cages, and water and diet were provided ad libitum. The experiment lasted for 21 d and the growth performance (average daily feed intake, average daily gain and gain-to-feed ratio) of chicks was analysed after the experiment. A total of six chicks from each of the four groups (one chick for each replicate) were weighed and slaughtered at 7, 14 and 21 d after hatching. Liver, duodenum, jejunum and ileum samples were collected immediately after slaughter and flash-frozen in liquid N2 for the determination of proinflammatory and anti-inflammatory cytokines.
Determination of proinflammatory and anti-inflammatory cytokines by real-time quantitative PCR
Total RNA was isolated from the frozen tissue (liver, duodenum, jejunum and ileum) of hens and chicks using a High Pure RNA Tissue Kit (Roche) following the manufacturer's recommendations. Total RNA was quantified using a spectrophotometer at an optical density of 260 nm (OD260), and the purity was assessed by determining the ratio of OD260 to OD280. This ratio ranged from 1·8 to 2·0 for all samples. Each total RNA sample was reverse-transcribed to complementary DNA using the ReverTra Ace qPCR RT kit (Toyobo) according to the manufacturer's instructions.
Quantitative real-time PCR analysis of IL-1β, IL-6, IFN-γ, LITAF, IL-4 and IL-10 mRNA was performed using the ABI 7500 Real-time PCR System (Applied Biosystems). Primer pairs were designed for IL-1β, IL-6, IFN-γ, LITAF, IL-4, IL-10 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) based on the published sequences with the following respective Genbank accession nos.: NM_204524, NM_204628, NM_205149, AY765397, NM_001007079, NM_001004414 and NM_204305, respectively. The forward and reverse primers for IL-1β were AGAAGAAGCCTCGCCTGGAT and CCGCAGCAGTTTGGTCAT; for IL-6: ATAAATCCCGATGAAGTGG and CTCACGGTCTTCTCCATAAA; for IFN-γ: TGAGCCAGATTGTTTCGA and ACGCCATCAGGAAGGTTG; for LITAF: TTCTATGACCGCCCAGTT and CAGAGCATCAACGCAAAA; for IL-4: GAGAGGTTTCCTGCGTCAAG and TGACGCATGTTGAGGAAGAG; for IL-10: CAATCCAGGGACGATGAAC and GCAGGTGAAGAAGCGGTGA; for GAPDH: ACTGTCAAGGCTGAGAACGG and CATTTGATGTTGCTGGGGTC, respectively.
Selection of a reference gene is of crucial importance for gene expression studies. GAPDH was selected as the reference gene because we found that many studies have used GAPDH as a housekeeping gene in the intestine and liver and used only one reference gene(Reference Theodoropoulos, Demers and Delvin23–Reference Pérez-Bosque, Miró and Polo27), although recent research has showed that housekeeping gene expression may vary in different tissues(Reference Ingerslev, Pettersen and Jakobsen28, Reference Martínez-Beamonte, Navarro and Larraga29) and use of a single gene for normalisation may lead to relatively large errors(Reference Vandesompele, De Preter and Pattyn30). A validation experiment was performed for each set of primers to confirm efficiency, amplification of a single gene and to optimise primer concentrations. PCR products from each gene were visualised by gel electrophoresis on 1 % agarose stained with ethidium bromide to ensure that a single product was produced of the predicted size. Single-band PCR products for each gene were sequenced and the products had 99 % homology to their respective gene transcripts. The 20 μl final PCR volume contained 1 μl RT product, 300 nmol/l forward and reverse primers and 10 μl 2 × concentrated SYBR Green Master mix (Roche). The PCR cycle was set at 95°C for 10 min followed by forty cycles of denaturing, annealing and extension at 95°C for 15 s and 58°C for 1 min (except at 56°C for LITAF). After the forty cycles were completed, a melting-curve analysis was performed to confirm that a single gene product was amplified. PCR data obtained from the ABI 7500 Real-time PCR System were automatically analysed by Applied Biosystems Software. The relative standard-curve method was used to quantify the mRNA concentrations of each gene in relation to the reference gene (GAPDH). Our approach was based on the calibrator-normalised relative quantification including correction for PCR efficiency. All samples were analysed in triplicate.
Statistical analysis
Statistical analysis of the data was performed using SAS 8.1 (SAS Institute Inc.). Fertilisation rate, hatchability of fertilised eggs, chick birth weight, healthy chick rate, and proinflammatory and anti-inflammatory cytokines at 0 d of chicks were analysed by t test between the two treatments. For analysing production performance, proinflammatory and anti-inflammatory cytokines of breeding hens, one-way ANOVA was performed. The dependent variable was examined for the main effect of dietary xanthophylls in hens. For the growth performance at 21 d of chicks, and proinflammatory and anti-inflammatory cytokines at 7, 14 and 21 d of chicks, data were analysed by using two-way ANOVA. The model included the main effects of in ovo, diet, and their interaction. Replicate was used as the experimental unit. Data are presented as means with their pooled standard errors. When the main effect(s) or interaction was significant, differences among means were determined using Tukey's honestly significant difference test. Differences among means were considered significant at P ≤ 0·05.
Results
Effects of xanthophylls on proinflammatory cytokine expression of hens
Supplementation of 20 or 40 mg/kg xanthophylls had no effect on fertilisation rate, hatchability of fertilised eggs, chick birth weight, healthy chick rate and production performance of hens (data not shown). Compared to the control group, addition of 20 or 40 mg/kg xanthophylls decreased IL-1β mRNA level in the liver (P = 0·028 and 0·008, respectively; Fig. 1) and jejunum (P = 0·032 and 0·037, respectively), IL-6 mRNA level in the liver (P = 0·007 and 0·004, respectively; Fig. 2), IFN-γ mRNA level in the jejunum (P = 0·040 and 0·015, respectively; Fig. 3) and LITAF mRNA level in the liver (P = 0·026 and 0·038, respectively; Fig. 4). In addition, the 40 mg/kg xanthophyll group also had reduced liver IFN-γ and duodenum LITAF mRNA level in contrast to the control group (P = 0·003 and 0·012, respectively).

Fig. 1 Effects of xanthophylls on relative IL-1β mRNA level in the liver, duodenum, jejunum and ileum of hens. Values are means, with their standard errors represented by vertical bars, n 6. a,b Mean values with unlike letters were significantly different (P < 0·05). P value (0·007, 0·139, 0·019 and 0·931) and pooled sem (1·13, 0·33, 0·33 and 0·23) from one-way ANOVA for liver, duodenum, jejunum and ileum, respectively. ![]() , Control;
, Control; ![]() , 20 mg/kg xanthophylls;
, 20 mg/kg xanthophylls; ![]() , 40 mg/kg xanthophylls.
, 40 mg/kg xanthophylls.

Fig. 2 Effects of xanthophylls on relative IL-6 mRNA level in the liver, duodenum, jejunum and ileum of hens. Values are means, with their standard errors represented by vertical bars, n 6. a,b Mean values with unlike letters were significantly different (P < 0·05). P value (0·002, 0·096, 0·157 and 0·327) and pooled sem (0·90, 0·45, 0·46 and 0·35) from one-way ANOVA for liver, duodenum, jejunum and ileum, respectively. ![]() , Control;
, Control; ![]() , 20 mg/kg xanthophylls;
, 20 mg/kg xanthophylls; ![]() , 40 mg/kg xanthophylls.
, 40 mg/kg xanthophylls.

Fig. 3 Effects of xanthophylls on relative interferon (IFN)-γ mRNA level in the liver, duodenum, jejunum and ileum of hens. Values are means, with their standard errors represented by vertical bars, n 6. a,b Mean values with unlike letters were significantly different (P < 0·05). P value (0·004, 0·150, 0·012 and 0·962) and pooled sem (1·32, 0·28, 0·14 and 0·14) from one-way ANOVA for liver, duodenum, jejunum and ileum, respectively. ![]() , Control;
, Control; ![]() , 20 mg/kg xanthophylls;
, 20 mg/kg xanthophylls; ![]() , 40 mg/kg xanthophylls.
, 40 mg/kg xanthophylls.
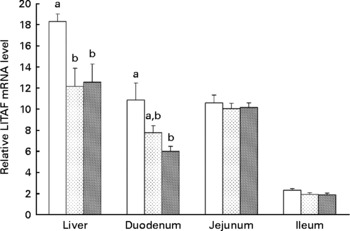
Fig. 4 Effects of xanthophylls on relative lipopolysaccharide-induced TNF-α factor (LITAF) mRNA level in the liver, duodenum, jejunum and ileum of hens. Values are means, with their standard errors represented by vertical bars, n 6. a,b Mean values with unlike letters were significantly different (P < 0·05). P value (0·017, 0·014, 0·779 and 0·148) and pooled sem (1·48, 1·04, 0·58 and 0·16) from one-way ANOVA for liver, duodenum, jejunum and ileum, respectively. ![]() , Control;
, Control; ![]() , 20 mg/kg xanthophylls;
, 20 mg/kg xanthophylls; ![]() , 40 mg/kg xanthophylls.
, 40 mg/kg xanthophylls.
Effects of xanthophylls on anti-inflammatory cytokine expression of hens
Supplementation of 40 mg/kg xanthophylls increased liver IL-10 mRNA level compared to the 20 mg/kg xanthophyll and control groups (P = 0·018 and 0·014, respectively; Fig. 5). There was no difference in IL-4 mRNA level among treatments (data not shown).

Fig. 5 Effects of xanthophylls on relative IL-10 mRNA level in the liver, duodenum, jejunum and ileum of hens. Values are means, with their standard errors represented by vertical bars, n 6. a,b Mean values with unlike letters were significantly different (P < 0·05). P value (0·008, 0·416, 0·219 and 0·974) and pooled sem (0·99, 0·46, 0·25 and 0·48) from one-way ANOVA for liver, duodenum, jejunum and ileum, respectively. ![]() , Control;
, Control; ![]() , 20 mg/kg xanthophylls;
, 20 mg/kg xanthophylls; ![]() , 40 mg/kg xanthophylls.
, 40 mg/kg xanthophylls.
Effects of xanthophylls on proinflammatory cytokine expression of chicks
Xanthophyll supplementation from in ovo, diet, or their interaction had no effect on the growth performance of chicks (data not shown). In ovo xanthophylls decreased liver IL-1β and IL-6 mRNA at 0 and 7 d, duodenum IL-6 and IFN-γ mRNA at 7 d and LITAF mRNA at 0 and 7 d, jejunum IL-1β mRNA at 0, 7 and 14 d and IL-6 mRNA at 0 d, and ileum IL-1β mRNA at 0 d and IFN-γ mRNA at 0 and 7 d (Tables 2–5). Dietary xanthophyll supplementation decreased liver IL-6 mRNA at 14 d and IFN-γ mRNA at 14 and 21 d, duodenum IL-6 and IFN-γ mRNA at 14 d, jejunum IL-1β mRNA at 14 and 21 d, and ileum IFN-γ mRNA at 21 d. LITAF mRNA in the liver, duodenum, jejunum and ileum were not affected by dietary xanthophylls.
Table 2 Effects of in ovo and dietary xanthophylls on relative IL-1β mRNA expression in the liver, duodenum, jejunum and ileum of chicks (Mean values with their pooled standard errors, n 6)
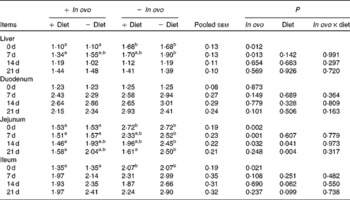
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 3 Effects of in ovo and dietary xanthophylls on relative IL-6 mRNA expression in the liver, duodenum, jejunum and ileum of chicks (Mean values with their pooled standard errors, n 6)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 4 Effects of in ovo and dietary xanthophylls on relative interferon-γ mRNA expression in the liver, duodenum, jejunum and ileum of chicks (Mean values with their pooled standard errors, n 6)
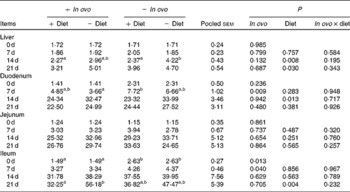
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 5 Effects of in ovo and dietary xanthophylls on relative lipopolysaccharide-induced TNF-α factor mRNA expression in the liver, duodenum, jejunum and ileum of chicks (Mean values with their pooled standard errors, n 6)
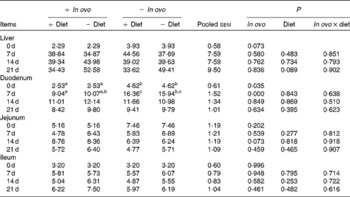
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Effects of xanthophylls on anti-inflammatory cytokine expression of chicks
Xanthophylls from in ovo increased liver IL-4 mRNA at 7 d and IL-10 mRNA at 0, 7 and 14 d, jejunum IL-4 and IL-10 mRNA at 0 and 7 d, and ileum IL-10 mRNA at 0 and 7 d (Tables 6 and 7). In ovo xanthophyll supplementation did not affect IL-4 and IL-10 mRNA in the duodenum. Dietary xanthophylls also enhanced liver IL-10 mRNA at 14 and 21 d, and jejunum IL-10 mRNA at 14 d. Addition of dietary xanthophylls had no effect on IL-4 mRNA in the liver, duodenum, jejunum and ileum.
Table 6 Effects of in ovo and dietary xanthophylls on relative IL-4 mRNA expression in the liver, duodenum, jejunum and ileum of chicks (Mean values with their pooled standard errors, n 6)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 7 Effects of in ovo and dietary xanthophylls on relative IL-10 mRNA expression in the liver, duodenum, jejunum and ileum of chicks (Mean values with their pooled standard errors, n 6)
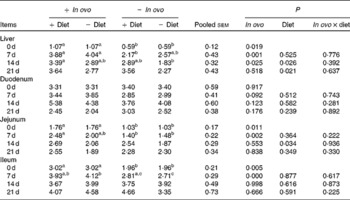
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
Measuring cytokine production is an integral part of measuring the immune response(Reference Siebert and Walker31). Cytokines can be generally divided into two types: proinflammatory (IL-1β, IL-6, IFN-γ, LITAF) and anti-inflammatory cytokines (IL-4, IL-10, TGF-β)(Reference Schiepers, Wichers and Maes32). Proinflammatory cytokines are essential for the development and functioning of both the innate and adaptive immune response, while their over-expression is associated with pathological conditions of the immune system(Reference Smith and Humphries33). Recently, researchers(Reference Bruewer, Luegering and Kucharzik34–Reference Ma, Iwamoto and Hoa37) found that high levels of TNF-α, IFN-γ, IL-1β and IL-6 could induce intestinal epithelial permeability. Research has shown that LITAF played an important role in the activation of TNF-α gene expression in mice(Reference Bolcato-Bellemin, Mattei and Fenton38) and humans(Reference Myokai, Takashiba and Lebo39). TNF has not been identified in chickens, whereas LITAF could induce the expression of TNFSF 15 (a member of the TNF ligand super family) and may play an important role in the regulation of TNF-α gene expression(Reference Hong, Lillehoj and Lee40). In Expt 1, a novel finding of the present study was that xanthophyll addition could decrease proinflammatory cytokine (IL-1β, IL-6, IFN-γ, LITAF) expression in the liver, duodenum and jejunum of hens, but not in the ileum. The same in vivo results were reported by research which showed that lutein supplementation reduced the concentrations of NO, TNF-α, IL-6, PGE2 and macrophage inflammatory protein-2 in the aqueous humor of endotoxin-induced uveitis in rats(Reference Jin, Ohgami and Shiratori9) and liver IL-1β mRNA in turkeys following lipopolysaccharide injection(Reference Shanmugasundaram and Selvaraj41). The inhibition of proinflammatory cytokines was also observed following the supplementation of other carotenoids in animals or cell lines, such as β-carotene in rats(Reference Pang, Wang and Weng10), astaxanthin in mice and rats(Reference Lee, Bai and Lee13, Reference Suzuki, Ohgami and Shiratori42), β-cryptoxanthin in murine macrophages(Reference Katsuura, Imamura and Bando11) and lycopene in human macrophages(Reference Simone, Russo and Catalano14, Reference Palozza, Simone and Catalano15).
Anti-inflammatory cytokines (IL-4, IL-10 and TGF-β) could prevent the over-activation of immune responses and the further production of proinflammatory cytokines and other mediators, thus mediating the balance between proinflammatory and anti-inflammatory responses(Reference Schiepers, Wichers and Maes32). Liver IL-10 mRNA of hens was increased by 40 mg/kg xanthophyll supplementation, indicating that xanthophylls could modulate anti-inflammatory cytokine expression. The effects of supplementing 40 mg/kg xanthophylls are better than 20 mg/kg xanthophylls based on our results that the 40 mg/kg xanthophyll group not only had reduced liver IFN-γ and duodenum LITAF mRNA but also elevated liver IL-10 mRNA level, which did not happen in the 20 mg/kg xanthophyll group. Xanthophylls did not affect proinflammatory and anti-inflammatory cytokine expression in the hen ileum as we measured, suggesting that the ileum is not the action site for xanthophylls. This may lie in the finding that xanthophylls, which are highly susceptible to oxidation, could be depleted before reaching the ileum. The main action site for xanthophylls may be in the liver because not only proinflammatory cytokine (IL-1β, IL-6, IFN-γ and LITAF) but also anti-inflammatory cytokine (IL-10) expression was modulated in the liver as compared to changed cytokine expression in the duodenum (LITAF) and the jejunum (IL-1β and IFN-γ).
Most yolk-derived carotenoids are deposited into the embryonic liver(Reference Speake, Murray and Noble43), but dietary carotenoids are more broadly distributed in the post-hatch chick(Reference Ganguly, Mehl and Deuel44). In Expt 2, we demonstrated that in ovo or dietary xanthophylls decreased proinflammatory cytokines in the liver, duodenum, jejunum and ileum. Furthermore, in ovo xanthophylls increased anti-inflammatory cytokines in the liver, jejunum and ileum, but not in the duodenum. Dietary xanthophylls also increased anti-inflammatory cytokines in the liver and jejunum, but not in the duodenum and ileum. Generally speaking, maternal xanthophylls modulated proinflammatory and anti-inflammatory cytokine expression mainly at 0–7 d after hatching. During 7–14 d after hatching, the maternal effects gradually disappeared and the progeny's diet began to take over. Dietary xanthophylls modulated cytokine expression mainly from 2 weeks onwards. The cytokine results were consistent with the liver carotenoid change of chicks as we (Table S1, supplementary material for this article can be found at http://www.journals.cambridge.org/bjn) and other researchers have determined(Reference Karadas, Pappas and Surai21). However, some studies revealed that lutein did not affect liver IL-6(Reference Meriwether, Humphrey and Peterson45) and the liver and spleen IL-1 mRNA of chicks following lipopolysaccharide challenge(Reference Selvaraj, Shanmugasundaram and Klasing46). The difference may be due to methodological diversity, such as environment (housing condition and density), dosage and type of carotenoids used, the interaction with other antioxidants (vitamin C, vitamin E) and immune challenge (our experiment did not include lipopolysaccharide challenge).
These changes of proinflammatory and anti-inflammatory cytokine expression may lie in the fact that immune cells are particularly sensitive to oxidative stress because their plasma membranes contain a high percentage of PUFA and they generally produce more reactive oxygen species(Reference Meydani, Wu and Santos47); carotenoids could help protect immune cells from oxidative damage(Reference Hughes48). For a precocial species like chicken, modulation of proinflammatory and anti-inflammatory cytokine expression may be helpful because the hatching process incurs sudden exposure to atmospheric concentrations of oxygen, and then there is a dramatic increase in the metabolic rate with the onset of pulmonary respiration and post-hatching growth(Reference Blount, Houston and Moller49). We also noted that the relative average value of IFN-γ mRNA in the duodenum, jejunum and ileum increased rapidly from 0 to 14 d after hatching, and reached a relatively steady level, onwards. The same change occurred to LITAF mRNA in the liver and duodenum from 0 to 7 d after hatching, and reached a relatively steady level, onwards. However, the average value of IL-4 mRNA decreased in the duodenum and jejunum after hatching. All of these changes may relate to a rapid increase of feed intake and microbial colonisation of the intestine after hatching. These data showed important developmental physiology of the chick by revealing that different parts of organs may have specific cytokine change as their sensitive indicators during chick development (LITAF for the liver; IFN-γ, LITAF and IL-4 for the duodenum; IFN-γ and IL-4 for the jejunum; IFN-γ for the ileum).
In conclusion, dietary xanthophyll supplementation decreased proinflammatory cytokine expression in the liver, duodenum and jejunum and increased anti-inflammatory cytokine expression in the liver of breeding hens. In ovo xanthophylls regulated proinflammatory cytokine (in the liver, duodenum, jejunum and ileum) and anti-inflammatory cytokine expression (in the liver, jejunum and ileum) mainly for at least the first week after hatching in chicks, whereas dietary xanthophylls played an important modulated role in proinflammatory cytokine (in the liver, duodenum, jejunum and ileum) and anti-inflammatory cytokine expression (in the liver and jejunum) mainly from 2 weeks onwards. Moreover, the determination of proinflammatory and anti-inflammatory cytokine expression in different organs (liver, duodenum, jejunum and ileum of chicks) at different times (0, 7, 14 and 21 d) may have special significance for deliberating the developmental physiology of chicks by indicating that different organs may have specific cytokine change during chick growth.
Acknowledgements
The present study was supported by Guangdong Natural Science Foundation of China (9151064201000052) and Guangdong Major Science and Technology Special Projects of China (2009B020201008). The contributions of the authors to this study were as follows: Y.-Y. G., Q.-M. X., L. J., J.-Y. M. and Y.-Z. B. designed the research; B.-L. S., J. J., F. C. and Y.-Z. B. conducted the research; Y.-Y. G. and Y.-Z. B. analysed the data; Y.-Y. G., Q.-M. X., J.-Y. M. and Y.-Z. B. wrote the paper. The authors declare that there are no conflicts of interest related to this paper.














