Fe is an essential trace element that is frequently deficient both in infants and in women of reproductive age from developing countries. Prevalence of anaemia is still high in rural areas in China(Reference Ma, Schouten and Wang1). Fe deficiency is associated with anaemia(Reference Schümann, Ettle and Szegner2) and impairment of Fe-dependent enzymes and proteins(Reference Beard, Dawson, O'Dell and Sunde3). Fe supplementation is almost universally recommended during pregnancy to correct or prevent deficiency(Reference Stoltzfus and Dreyfuss4). However, the pathological accumulation of the metal within the tissues aggravates the generation of reactive oxygen species and elicits toxic effects, which are mainly related to oxidative stress(Reference Galaris and Pantopoulos5). Fe plays a central role in generating harmful oxygen species. Its redox cycling promotes the Fenton reaction, producing the potent oxidant hydroxyl radical(Reference Halliwell and Gutteridge6). Reactive oxygen species can alter the chemical and physical properties of cell membranes leading to a structural alteration, which could modify membrane activity and cause a reduction in membrane fluidity that can be assessed in the erythrocyte(Reference Lutz7). Several studies have reported that free radicals, including chemical scavengers or antioxidant molecules, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), are relatively low in normal conditions due to active defence systems. In rats, both Fe deficiency and excess result in free radical mitochondrial damage(Reference Srigiridhar, Nair and Subramanian8). The excessive superoxide produced by abnormal redox reactions can also cause biochemical modification in erythrocyte membrane proteins. This could result in changes in membrane structure or trans-membrane transport that may exert a variety of cytotoxic effects(Reference Uyesaka, Hasegawa and Schechter9), and affect membrane viscoelasticity.
In relation to pregnancy, there was a report that supplementation with vitamin C (1000 mg/d) and vitamin E (400 mg/d) might be beneficial in pregnant women with increased risk of pre-eclampsia, a complication in which oxidation is an important feature(Reference Chappell, Seed and Briley10). Two large randomised trials showed, however, that supplementation with vitamins C and E during the second trimester did not reduce the pre-eclampsia(Reference Poston, Briley and Seed11, Reference Rumbold, Crowther and Haslam12). The administration of an Fe supplement with vitamin C to twenty-seven women during the third trimester of pregnancy significantly increased maternal Fe status as well as an indicator of lipid peroxidation, compared with controls(Reference Lachili, Hininger and Faure13). Increased oxidative stress was reported to occur in uncomplicated pregnancy and to be counteracted by a high level of plasma vitamin E(Reference Cargnoni, Gregorini and Ceconi14).
Thus, provision of a moderate dose of Fe (100 mg/d) might be deleterious in certain circumstances and not in others, with respect to the possible generation of reactive oxygen species. Therefore, the purpose of the study was to investigate the effect of supplementation with Fe only and Fe combined with folic acid or with folic acid, retinol and riboflavin on haemotological status, oxidative stress parameters, such as SOD, GSH-Px and malondialdehyde (MDA) level, and erythrocyte membrane fluidity in anaemic pregnant women during the trial.
Materials and methods
Participants
The study was a 2-month intervention trial conducted on the effect of (1) Fe, (2) Fe and folic acid and (3) Fe, folic acid, retinol and riboflavin on indicators of oxidative stress, compared with placebo. Participants were recruited between March 2004 and September 2006 from the community hospitals of Shen County in the central area of China. A total of 366 pregnant women consented to participate and fulfilled the other eligibility criteria of having taken no dietary supplements during the past 2 months and having no abnormal pregnancy response. Finally, a random sample of 164 anaemic pregnant women (80 g/l < Hb < 110 g/l), 12–24 weeks gestation, and 20–35 years old, were randomly allocated to four groups in the order of recruitment: group C (n 41) was the placebo control group, group I (n 41) was supplemented daily with 60 mg Fe as ferrous sulphate, group IF (n 41) was supplemented daily with 60 mg Fe and 400 μg folic acid, group IM (n 41) was supplemented daily with 60 mg Fe, 400 μg folic acid, 2 mg retinol and 1 mg riboflavin. The sample size was calculated based on a difference in Hb change (δ) of 5 g/l between the intervention and placebo groups, and a standard error of 6·67 g/l was derived from the study reported by Suharno et al. (Reference Suharno, West and Karyadi15). Considering a 5 % (α = 0·05) significance level and a power of 0·80 (β = 0·20), the total number of subjects required for the study was 164. Treatments were blindly assigned to the groups, leaving the key in a sealed envelope with an independent person in the institute. The capsules were labelled in red, yellow, green and blue colour, and manufactured by Hurun company (a Chinese food-additives company, Beijing, China). Trial participants and the research team were unaware of the treatment assignment. The trial was unblinded after analysis of the primary outcomes.
Pregnant women were enrolled after obtaining written informed consent and had a baseline interview on characteristics, such as age, gestation, and previous pregnancies. In each community, a local female community health worker called ‘village nurse’ was responsible for the recruitment and distribution of the supplements. After ascertainment of eligibility, consenting women were enrolled in the study, had a baseline interview and started with their allocated supplements to be taken daily for a period of 2 months. Women were home-visited once a week by the village nurse to replenish supplements and to monitor compliance by counting and recording the number of supplements that were taken. The nurse also provided counselling about the possible side effects.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects were approved by the ethics committee of Medical College of Qingdao University (QY-ETC2002821).
Sample collection and laboratory analyses
Before and at the end of the intervention, overnight fasting (>12 h) blood samples were collected between 6.00 and 8.00 hours. Moreover, daily sample collection was evenly distributed over each of the four groups. The urine first passed was discarded, and the next 10 ml was collected in dark containers, which were immediately aliquoted, for riboflavin and creatinine analysis. The samples were transported on dry-ice and stored frozen at − 80°C until analysis. The baseline and final samples were analysed in duplicate during the same analytic run.
Plasma retinol concentrations were measured by reversed-phase HPLC (Beckman 5000 with detector of 168; Fullerton, CA, USA), and the within-assay and between assay CV were 3 and 8 %, respectively(Reference Handelman, Shen and Krinsky16). Folic acid in plasma was measured by the RIA method. The nutritional status of riboflavin was determined by the ratio of urine riboflavin:creatinine(Reference Sauberlich, Judd and Nichoalds17).
Hb concentration was measured by the cyanomethaemoglobin method by using HemoCue for confirmation. The cutoff value of anaemia was Hb < 110 g/l. Measurements of serum ferritin were performed by RIA(Reference Liu, Han and Zhang18), as described by the manufacturer (The North Biological Technology Institute, Beijing, China). Plasma Fe concentrations were analysed by atomic absorption spectrometry on an Analyst 3100 Analyser (Perkin Elmer Life Sciences, Wellesley, MA, USA).
The activities of SOD and GSH-Px were determined in plasma(Reference Durak, Canbolat and Kavutcu19) as U/ml and IU/ml, respectively. The MDA concentration was determined by using the thiobarbituric acid reaction, and calculated by comparing the absorbance values of the samples with those of standard MDA solutions. Results were expressed as nmol/ml blood. Additionally, routine parameters were also studied in plasma from the subjects(Reference Avci, Atli and Ergüder20).
The erythrocyte membrane fluidity can be measured by fluorescence polarisation (ρ) and microviscosity (η). To evaluate membrane fluidity by fluorescence spectroscopy, cell suspensions were incubated with the fluorescence probes (final concentration 10− 6 m). Erythrocytes were washed with the physiological saline, and suspended in the PBS solution (pH = 7·4) at 0·01 mol/l. To the erythrocytes suspension, 1,6-diphenyl-1,3,5-hexatriene (2 × 10− 6 mol/l) was added before being incubated in a water-bath at 25°C for 15 min(Reference Xue, Hou and Zhang21). Samples were illuminated with the linear (vertically – V or horizontally – H) polarised monochromatic light (λex), and the emitted fluorescence intensities (I – in arbitrary units) parallel or perpendicular to the direction of the excitation beam were recorded. The suspension was examined by spectrofluorophotometer (Perkin Elmer fluorescence spectrometer, LS-50) with the excitation wavelength of 320 nm and emission wavelength of 430 nm(Reference Sun, Ma and Zhang22, Reference Ortiz, Pacheco-Moisés and El Hafidi23); ρ and η of the erythrocyte membrane were calculated by the following formulae:
where the indices V and H denote the vertical and horizontal position of the polariser in the excitation and the fluorescence beams, respectively. G is an instrumental correction factor equal to (I VV/I VH). The subscript H refers to the horizontally polarised excitation beam. I VV and I VH represent the components of the corrected polarised emission parallel and perpendicular to vertical direction, respectively(Reference Tang, Xia and Yu24, Reference Marczak25).
Statistical analysis
Categorical data are presented as frequencies, such as prevalence of anaemia. Continuous data, haematological indicators, vitamin concentrations, oxidative parameters, η and ρ values were normally distributed and presented as means and standard deviations. Baseline variables were compared across the treatment groups using a general linear model ANOVA. Mean changes over the intervention period and differences between the groups were tested with Student t tests for haematological indicators, vitamin levels, oxidative indicators, η and ρ values. A two-sided P < 0·05 was considered as the significant level for all tests.
Results
Complete data were available for 145 of the original 164 pregnant women. Nineteen women did not complete the trial for the following reasons: five women were moved to other villages, eleven women were stopped taking supplements and three women were not willing to provide a second blood sample. There were no substantial differences between the remaining groups in any of the baseline characteristics (Fig. 1 and Table 1). Compared with the means of haematological status at the start of the trial, significant decreases of Hb, plasma Fe and ferritin values were found in the C group 2 months later, whereas the levels of haematological status were significantly increased in the I, IF and IM groups after 2 months supplementation (Fig. 2).
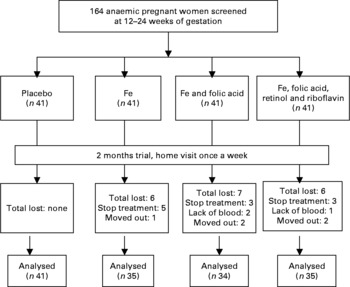
Fig. 1 The trial profile. The trial enrolment was conducted from 2004 to 2006. A total of 366 women were eligible, from whom we took a random sample of 164, who were allocated to the intervention groups by order of randomisation. In the intervention study, complete data were available on 145 pregnant women, which is 88·4 % of the original number of 164 pregnant women. Nineteen women did not complete the trial. However, there were no substantial differences between the groups in any of the baseline characteristics.
Table 1 Baseline characteristics of Chinese anaemic pregnant women in four groups*
(Mean values and standard deviations)
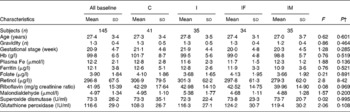
* Group C, placebo; group I, 60 mg ferrous sulphate; group IF, in addition 400 μg folic acid; group IM, in addition 2 mg retinol and 1 mg riboflavin.
† One-way ANOVA.
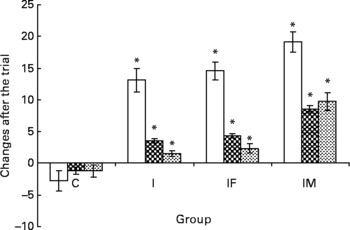
Fig. 2 A comparison of haematological status between before and after the trial. □ represent the mean and standard deviation of Hb concentration, ![]() for plasma iron concentration and
for plasma iron concentration and ![]() for ferritin with significant changes between groups; group C as placebo, group I supplemented daily with 60 mg iron, IF with 60 mg iron and 400 μg folic acid and IM with 60 mg iron, 400 μg folic acid, 2 mg retinol and 1 mg riboflavin, respectively. * Mean values were significantly different from that of the control group (P < 0·05).
for ferritin with significant changes between groups; group C as placebo, group I supplemented daily with 60 mg iron, IF with 60 mg iron and 400 μg folic acid and IM with 60 mg iron, 400 μg folic acid, 2 mg retinol and 1 mg riboflavin, respectively. * Mean values were significantly different from that of the control group (P < 0·05).
After the 2 months supplementation, there were considerable increases in haematological indicators in the I, IF and IM groups compared with the C group: 15·8, 17·3 and 21·8 g/l for Hb (all P values < 0·001); 4·7, 5·5 and 9·7 μmol/l for plasma Fe (all P values < 0·001); 2·8, 3·6 and 11·0 μg/l for ferritin (all P values < 0·001). The increases of plasma retinol and urine riboflavin in the IM group were 267·4 μg/l (P < 0·001)) and 61·59 mg/g creatinine (P < 0·001), respectively. For plasma folic acid, the increase was 4·06 μg/l in the IF group (P < 0·001) and 4·65 μg/l in the IM group (P < 0·001), all compared with the C group (Table 2). The increases in all haematological and vitamin indicators in the IM group were significantly greater than in the I group (all P values < 0·001).
Table 2 Comparison of haematological and vitamin status between control and supplemented groups* after the trial
(Mean values and standard deviations)
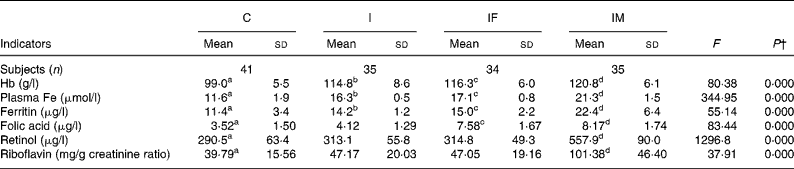
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05, independent t test).
* Group C, placebo; group I, 60 mg ferrous sulphate; group IF, in addition 400 μg folic acid; group IM, in addition 2 mg retinol and 1 mg riboflavin.
† One-way ANOVA.
Erythrocyte membrane fluidity was evaluated using fluorescence ρ and η; lower values indicate better membrane fluidity. After the trial, the ρ and η values, compared with the C group, decreased by 0·033 and 0·959 for the I group, 0·037 and 1·074 for the IF group, and 0·064 and 1·865 for the IM group, respectively. The ρ and η values in the IM compared with those in the I group decreased by 0·031 and 0·906 (all P values < 0·05) (Table 3).
Table 3 Comparison of membrane fluidity and oxidative stress status between control and supplemented groups* after the trial
(Mean values and standard deviations)
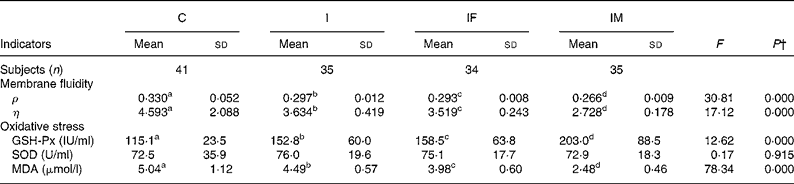
ρ, Polarisation; η, microviscosity; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05; independent t test).
* Group C, placebo; group I, 60 mg ferrous sulphate; group IF, in addition 400 μg folic acid; group IM, in addition 2 mg retinol and 1 mg riboflavin.
† One-way ANOVA.
The changes in oxidative stress parameters are presented in Table 3. Compared with the C group, increases of GSH-Px activities were 37·7, 43·4 and 87·9 IU/ml in the I, IF and IM groups, respectively; the levels of MDA decreased by 0·55, 1·06 and 2·56 μmol/l. Moreover, the increase of GSH-Px activity and decrease of MDA level were 50·2 IU/ml and 2·01 μmol/l greater in the IM group compared with the I group (all P values < 0·01); however, there were no important changes in SOD activities during the trial.
Discussion
After 2 months of supplementation with Fe and/or vitamins, anaemic pregnant women significantly improved in erythrocyte membrane fluidity and antioxidative markers, in addition to increases of Hb concentration, plasma Fe, ferritin, retinol and urine riboflavin concentrations. Most of the effects were greatest in the Fe combined with multivitamin (IM) group.
Up to now, only few studies have evaluated the changes in both haematological status and erythrocyte membrane fluidity after Fe supplementation in anaemic pregnant women, possibly because erythrocyte membrane preparation is quite laborious. The present study had a relatively large study population and it was carried out under difficult circumstances in a poor rural part of China. Unfortunately, there was a small proportion of women who dropped out after treatment allocation, only in the supplement groups. Some subjects stopped treatment because of possible side effects, including nausea, abdominal discomfort and vomiting(Reference Ma, Schouten and Zhang26). For other subjects, data were not complete because of an insufficient amount of plasma, subjects refusing to give a second blood sample or they were moving out of the area. This did not result in imbalances with respect to important baseline characteristics. Compliance of the remaining participants was almost complete, probably because the study subjects were motivated by the offer of free of charge medical care, and bi-weekly visits by village nurses.
In fact, retinol and riboflavin deficiencies tend to coexist in anaemic pregnant women in developing countries or in impoverished settings(Reference Bates, Prentice and Paul27). In a previous study(Reference Ma, Schouten and Wang1), high percentages of subnormal vitamin A and subnormal riboflavin were 23 and 38 % in anaemic pregnant women. Vitamin A deficiency may also result in anaemia in human subjects and animals that can be reversed only by vitamin A supplementation(Reference Bloem, Wedel and Egger28). Biochemical evidence of riboflavin deficiency was documented during the third trimester of pregnancy(Reference Pongpaew, Saowakontha and Schelp29). Retinol or riboflavin plus Fe supplementation could improve haematological status better than Fe alone(Reference Suprapto, Widardo and Suhanantyo30). The reduced prevalence of anaemia (Hb < 110 g/l) and Fe-deficiency anaemia was significantly greater in the groups supplemented with retinol and/or riboflavin than in the groups supplemented with Fe. Retinol status is a putative factor for improved Fe status or Fe absorption. Vitamin A and β-carotene may form a complex with Fe, keeping it soluble in the intestinal lumen as well as preventing the inhibitory factors on Fe absorption(Reference Semba and Bloem31). Moreover, gastrointestinal symptoms were less prevalent in the group supplemented with Fe, folic acid and retinol than in the group supplemented with Fe only, and improved well-being was more prevalent in the groups that received additional retinol and/or riboflavin than in the group that received Fe only.
Although fluorescence ρ was used to assess membrane fluidity in the past decades, presently, it is frequently applied in estimating oxidative stress(Reference Soto-Arriaza, Sotomayor and Lissi32), erythrocyte aggregation(Reference Spengler, Bertoluzzo and Catalani33), understanding of membrane protein function(Reference Periasamy, Teichert and Weise34), etc. The noninvasiveness of the method makes it especially suitable for clinical applications(Reference Kaneko, Matsui and Shimokawa35). Pregnancy is a condition exhibiting increased susceptibility to oxidative stress. In normal pregnancy, the implantation process, proliferation, differentiation and trophoblast invasion produce reactive oxygen species(Reference Gitto, Pellegrino and Gitto36, Reference Dennery37). Transitional metals, especially Fe, which are particularly abundant in the placenta, are important in the production of these free radicals(Reference Casanueva and Viteri38). Increased Fe levels may be responsible for placental oxidative stress and abnormalities in antioxidants(Reference Vaughan and Walsh39). Fe overload could promote the generation of free radicals and result in cellular damage(Reference Rumbold, Crowther and Haslam12), and Fe supplementation with 100 mg/d or overload might risk increased MDA levels or oxidative stress in the maternal plasma and the placenta during pregnancy(Reference Devrim, Tarhan and Ergüder40), but in a previous study, low doses of oral ferrous Fe (36 mg/d) did not unfavourably change the physiological pattern of parameters of oxidation(Reference Rehema, Zilmer and Klaar41). However, in the present study, haemotological status was improved and MDA levels decreased in anaemic pregnant women after a daily supplementation of 60 mg Fe, which might be attributed to the supplementation with a low dose of Fe and a combination of three vitamins. Erythrocyte membrane fluidity also improved, which indicates that the Fe supplementation did not reduce, but possibly even improved, the antioxidant capacity. There are no documented side effects of Fe supplements below 100 mg/d(Reference Milman42). In the present study, Fe combined with multivitamin supplementation showed a more favourable effect than Fe alone, and this dose of Fe supplementation did not increase Hb levels higher than the optimal concentration needed for O2 delivery(Reference Yip43). A daily supplementation of 100 mg Fe as ferrous sulphate was recommended during the second half of pregnancy to address the corresponding Fe requirements(Reference Schümann, Ettle and Szegner2), and the risks associated with oxidative stress were not observed in women supplemented with 120 mg Fe once or twice per week(Reference Casanueva and Viteri38). Suggested guidelines are ferritin < 30 μg/l: 80–100 mg ferrous Fe daily, for which there are no documented side effects(Reference Milman42).
Several studies have shown that micronutrient supplementation improves the GSH-Px activities and decreases the levels of MDA(Reference Avci, Atli and Ergüder20, Reference Zhang, Ma and Zhang44), and antioxidant supplementation was associated with better maternal and perinatal outcome in pregnant women with low antioxidant status than supplementation with Fe and folate alone(Reference Rumiris, Purwosunu and Wibowo45). However, we did not find any change of SOD activity during the trial. Some studies showed similar results(Reference Oh, Lim and Lee46) and even a decrease of SOD activity after antioxidant supplementation(Reference Ahmed, Suke and Seth47). This aspect deserves further study. In the present study, antioxidant defences and oxidative stress appear to be favourably modulated by Fe supplementation alone. Such findings have not been reported in anaemic pregnant women before, and further mechanistic studies are to be expected.
Taken together, subjects had more antioxidative capacity, i.e. lower levels of circulating lipid peroxidation products (MDA), and higher GSH-Px activity and higher vitamin concentrations after 2 months of supplementation. The improvement of erythrocyte membrane fluidity, as apparent in decreases of ρ and η, indicates that the erythrocytes accumulate fewer oxidative lesions. Moreover, the increase in the plasma antioxidant status could contribute to the prevention of PUFA peroxidation of erythrocytes. This evidence could also explain the lower peroxidation found in subjects supplemented with Fe and vitamins in comparison with the control. Fe combined with multivitamin supplementation showed a more favourable effect than Fe only. We could not find any indication of an increase of oxidative stress after Fe supplementation. On the contrary, a moderate dose of Fe, preferably combined with multivitamin supplementation, may be beneficial both by improving anaemia and by decreasing the status of oxidative stress during pregnancy.
Acknowledgements
We thank the Nestlé Foundation and the Danone Nutrition Institute, China, for financial support. A. G. M. and E. G. S. designed the intervention study. Y. Y. S., F. Y., F. Z. Z. and D. C. J. conducted investigation in field sites, data collection, analyses and interpretation and laboratory analyses. X. X. H. analysed the data. A. G. M., E. G. S. and F. J. K. wrote the paper. There are no conflicts of interest.







