Consumption of sugar-sweetened beverages (SSB), including soft drinks, fruit drinks, iced tea, and energy and vitamin water drinks, has increased in the USA and Europe over the past three decades( Reference Nielsen and Popkin 1 ). A higher consumption of SSB has been associated with weight gain, obesity( Reference Te Morenga, Mallard and Mann 2 ), the metabolic syndrome and diabetes( Reference Greenwood, Threapleton and Evans 3 ), which may be attributed to their high energy and sugar content and lack of nutrients.
To date, a few studies have investigated the associations between SSB consumption and the risk of hypertension, CHD and stroke( Reference Dhingra, Sullivan and Jacques 4 – Reference Larsson, Akesson and Wolk 13 ). However, the results have been inconsistent, with some reporting a positive association and others finding no relationship. Although two recent systematic reviews( Reference Malik, Popkin and Bray 14 , Reference Malik, Akram and Shetty 15 ) have commented on the associations of SSB consumption with the risk of CVD and hypertension, they did not quantify the associations using a meta-analysis. Thus, it is still unclear whether SSB consumption is associated with cardiovascular risk.
In the present study, we performed a systematic review and meta-analysis of prospective cohort studies to clarify the dose–response associations between SSB consumption and the risk of hypertension, CHD and stroke.
Materials and methods
Literature and search strategy
The Meta-analysis of Observational Studies in Epidemiology guidelines were followed for the present study( Reference Stroup, Berlin and Morton 16 ). A literature search was performed using databases including PubMed and Embase. The search terms included ‘sugar-sweetened beverages’ (or ‘soft drink’ or ‘soft drinks’ or ‘beverage’ or ‘beverages’ or ‘carbonated soft drinks’ or ‘fruitades’ or ‘fruit drinks’ or ‘sports drinks’ or ‘energy and vitamin water drinks’ or ‘sweetened iced tea’ or ‘punch’ or ‘fruit punch’ or ‘cordials’ or ‘squashes’ or ‘lemonade’ or ‘soda’ or ‘soda-pop’); ‘hypertension’ (or ‘HBP’ or ‘high blood pressure’ or ‘blood pressure’); ‘coronary heart disease’ (or ‘CHD’ or ‘angina’ or ‘ischemic heart disease’ or ‘IHD’ or ‘myocardial ischemia’ or ‘myocardial infarction’ or ‘MI’ or ‘coronary artery disease’ or ‘atherosclerosis’ or ‘cardiovascular disease’ or ‘CVD’ or ‘vascular disease’ or ‘vascular event’); ‘stroke’ (or ‘ischemic stroke’ or ‘cerebral infarction’ or ‘cerebrovascular disease’); and ‘prospective’ (or ‘cohort’ or ‘follow up’ or ‘following’ or ‘longitudinal’ or ‘incidence’). The search strategy is given in detail in the online Supplementary material. The search was limited to studies carried out in human subjects only. The reference lists of retrieved articles were also screened. The literature search was limited to the English language. If more than one article was published on the same cohort, only the study with the largest sample size was included. The literature search was updated on 5 May 2014.
Inclusion criteria and data extraction
Studies included in the meta-analysis met all the following inclusion criteria: (1) evaluated any association between SSB consumption and the risk of hypertension, CHD or stroke; (2) used a prospective cohort design; (3) provided the amount of SSB consumption, distributions of cases and person-years, and relative risks (RR) or hazard ratios (HR) with 95 % CI for at least three exposure categories. The following information was extracted from each study: (1) name of the first author; (2) year of publication; (3) country of study; (4) sex of participants; (5) age distribution of the study population at baseline; (6) average duration of follow-up; (7) number of cases and study population; (8) outcome; (9) RR or HR with 95 % CI for all categories of SSB consumption; (10) covariates used in adjustment. To assess the compliance with the inclusion/exclusion criteria, two authors (B. X. and D. Z.) independently searched and assessed the abstracts and full-text articles. If there was a discrepancy in the screening decision, a third investigator was asked to discuss and resolve it.
The quality of each study was assessed by the Newcastle–Ottawa quality scale (see online Supplementary Table S1)( Reference Wells, Shea and O'Connell 17 ), which is a validated scale for non-randomised studies in meta-analyses. This scale assigned a maximum of nine points for each study. The following three broad perspectives are considered: selection of cohorts (four points); comparability of cohorts (two points); ascertainment of the exposure and outcome of interest (three points).
Statistical analyses
Fixed-effects( Reference DerSimonian and Laird 18 ) or random-effects( Reference Mantel and Haenszel 19 ) models, selected on the basis of whether there was a between-study heterogeneity, were used to calculate the pooled RR with 95 % CI for the highest compared with the lowest category of SSB consumption. Dose–response analyses were also conducted. The Q test and the I 2 statistic( Reference Higgins, Thompson and Deeks 20 ) were used to examine between-study heterogeneity. P< 0·10 for the Q test or I 2>50 % represented significant heterogeneity, and random-effects models were used when significant heterogeneity was present; otherwise, fixed-effects models were used.
The generalised least-squares trend estimation, reported by Greenland & Longnecker( Reference Greenland and Longnecker 21 ) and Orsini et al. ( Reference Orsini, Bellocco and Greenland 22 ), was used to calculate study-specific slopes (linear trends) for the dose–response analyses based on the results across the categories of SSB consumption. We extracted data on the amount of SSB consumption, the distribution of cases and person-years, and RR with 95 % CI for at least three categories of exposure to SSB. The definition of the median or mean level of SSB consumption in each category of the included studies has been described elsewhere( Reference Rong, Chen and Zhu 23 ). The dose–response results in the forest plots are presented for every one serving/d increment in SSB consumption. Doses reported as servings/week (or month) were converted to servings/d. For example, one serving/week is equal to 1/7, which is approximately 0·143 servings/d. Doses reported as cups/d (or week or month) were treated as servings/d (or week or month), although this may introduce some small degree of inaccuracy. A four-knot restricted cubic spline model was applied to obtain three spline transformations of aggregated SSB intakes. Then, the restricted cubic spline model was nested within the generalised least-squares trend model to obtain the P value for non-linearity. We tested the joint null hypothesis that the regression coefficients of the last two spline transformations were all equal to zero( Reference Orsini, Bellocco and Greenland 22 , Reference Orsini, Li and Wolk 24 ). If the test for the non-linear association was not significant, the simple generalised least-squares trend model without the restricted cubic spline model was used to test the linear hypothesis.
To examine the stability of the present results, we performed influence analysis by exclusion of one study at a time. Meta-regression analyses were used to examine the potential sources of heterogeneity between studies. Begg's test( Reference Begg and Mazumdar 25 ) and Egger's test( Reference Egger, Davey Smith and Schneider 26 ) were used to examine publication bias. P< 0·05 represented statistical significance. All statistical analyses were performed using STATA version 12 (StataCorp LP).
Results
Characteristics of the included prospective studies
The results of the literature search are shown in Fig. 1. If the original publications provided several independent studies, they were considered as separate studies in the following data analysis. The duration of follow-up ranged from 6 to 38 years for hypertension, 10 to 24 years for CHD, and 10 to 28 years for stroke. The characteristics of the included prospective studies are listed in Table 1 and online Supplementary Table S1.

Fig. 1 Flow diagram of the literature search and study selection.
Table 1 Characteristics of the included prospective studies examining the association between sugar-sweetened beverage consumption and the risk of hypertension, CHD and stroke

SBP, systolic blood pressure; DBP, diastolic blood pressure; NHS, Nurses' Health Study; HPFS, Health Professionals Follow-Up Study.
Sugar-sweetened beverage consumption and risk of hypertension
Highest v. lowest intake
A total of six prospective studies from four publications, including 240 726 participants and 80 411 incident cases of hypertension, were included in the meta-analysis. The highest intake of SSB was positively associated with the risk of hypertension (random-effects model: RR 1·10, 95 % CI 1·06, 1·15, P< 0·001; Fig. 2(a)) compared with the lowest level, with significant evidence of heterogeneity (I 2= 46·7 %, P= 0·095). In the sensitivity analyses, RR were stable, ranging from 1·11 (95 % CI 1·06, 1·16) to 1·16 (95 % CI 1·08, 1·24). There was no evidence of publication bias as revealed by Begg's test (P= 0·71) and Egger's test (P= 0·25).

Fig. 2 Meta-analysis of the association between sugar-sweetened beverage consumption (highest v. lowest) and the risk of incident (a) hypertension, (b) CHD and (c) stroke.
Dose–response analysis
The test for the non-linear association between SSB consumption and the risk of hypertension was not significant (P for non-linearity = 0·22; Fig. 3(a)). Under the linear hypothesis, a higher consumption of SSB was significantly associated with an increased risk of hypertension (summary RR 1·08, 95 % CI 1·04, 1·12). An increase in one serving/d was associated with a higher risk of developing hypertension (P for trend < 0·05).
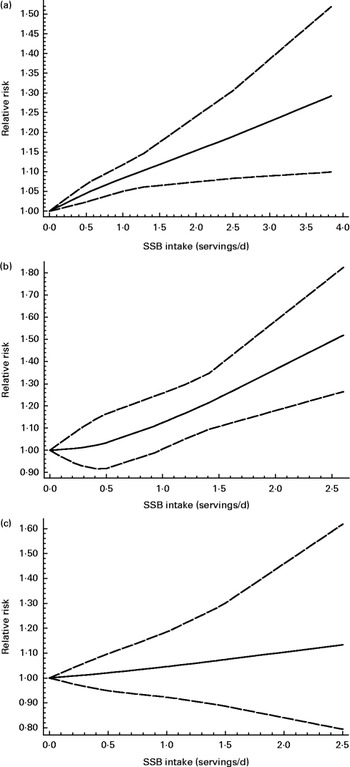
Fig. 3 Dose–response association between sugar-sweetened beverage (SSB) consumption and the risk of incident (a) hypertension, (b) CHD and (c) stroke (for every serving/d increase). ![]() , Best-fitting restricted cubic spline;
, Best-fitting restricted cubic spline; ![]() , 95 % CI.
, 95 % CI.
Sugar-sweetened beverage consumption and risk of CHD
A total of four prospective studies from four publications, including 194 664 participants and 7396 incident cases of CHD, were included in the meta-analysis. An apparent significant association between SSB intake and the risk of CHD was found (RR 1·16, 95 % CI 1·06, 1·27) for the highest compared with the lowest consumption of SSB in the fixed-effects model (P< 0·001; Fig. 2(b)), with no evidence of heterogeneity (I 2= 0 %, P= 0·792). These results were stable after excluding each study at one time, with RR ranging from 1·13 (95 % CI 0·94, 1·35) to 1·18 (95 % CI 1·07, 1·30). There was no evidence of publication bias as revealed by Begg's test (P= 0·31) and Egger's test (P= 0·30).
The subgroup analysis by race suggested that there was a significant association between SSB intake and the risk of hypertension in Caucasians (RR 1·18, 95 % CI 1·07, 1·30), but not in East Asians (RR 1·02, 95 % CI 0·74, 1·42).
Dose–response analysis
The test for the non-linear association between SSB consumption and the risk of hypertension was not significant (P for non-linearity = 0·82; Fig. 3(b)). A higher consumption of SSB was significantly associated with an increased risk of CHD. For example, one serving/d increase in SSB consumption relatively increased the risk of developing CHD by 17 % (RR 1·17, 95 % CI 1·10, 1·24, P for trend < 0·001).
Sugar-sweetened beverage consumption and risk of stroke
A total of four prospective studies from four publications, including 259 176 participants and 10 011 incident cases of stroke, were included in the meta-analysis. The highest intake of SSB was marginally associated with the risk of total stroke (fixed-effects model: RR 1·10, 95 % CI 1·00, 1·20, P< 0·05; Fig. 2(c)) compared with the lowest level, with little evidence of heterogeneity (I 2= 43·4 %, P= 0·151). However, these results were not stable after exclusion of each study at one time, with RR ranging from 1·07 (95 % CI 0·95, 1·20) to 1·17 (95 % CI 1·06, 1·28). There was no evidence of publication bias as revealed by Begg's test (P= 0·31) and Egger's test (P= 0·38).
The subgroup analysis by types of stroke suggested that there were no significant associations between SSB intake and the risk of either ischaemic stroke (RR 1·16, 95 % CI 0·93, 1·46) or haemorrhagic stroke (RR 0·86, 95 % CI 0·71, 1·04). However, SSB intake was associated with a higher risk of stroke in Caucasians (RR 1·17, 95 % CI 1·06, 1·28), but not in East Asians (RR 0·92, 95 % CI 0·75, 1·13).
Dose–response analysis
The test for the non-linear association was not significant (P for non-linearity = 0·82; Fig. 3(c)). There was no significant association between SSB consumption and the risk of stroke (summary RR 1·06, 95 % CI 0·97, 1·15, P for trend >0·05).
Between-study heterogeneity
To examine the potential sources of heterogeneity between the included studies for hypertension outcome, meta-regression analyses were performed with the following independent variables: sex; age; origin of country; sample size of the studies; mean BMI; mean age of the populations; whether adjustment for confounders was made or not; sex proportion of the participants; length of follow-up; study quality. However, none of these variables was identified as a relevant source of heterogeneity, suggesting that other unknown or unmeasured factors might be responsible for the observed heterogeneity in the association between SSB intake and the risk of hypertension.
Discussion
To our knowledge, this is the first quantitative meta-analysis investigating the association between SSB consumption and the risk of hypertension, CHD and stroke. In the present meta-analysis, a higher consumption of SSB was associated with an increased risk of hypertension and CHD, but not with the risk of stroke. These observed associations were independent of dietary and lifestyle factors, such as BMI or energy intake.
Comparison with other studies
Several meta-analyses have addressed the association between SSB consumption and the risk of obesity( Reference Te Morenga, Mallard and Mann 2 , Reference Forshee, Anderson and Storey 27 ), the metabolic syndrome, type 2 diabetes( Reference Greenwood, Threapleton and Evans 3 ), pancreatic cancer( Reference Gallus, Turati and Tavani 28 ) and colon cancer( Reference Zhang, Albanes and Beeson 29 ). Because SSB include high energy and sugar content, it is not surprising that SSB consumption may be significantly associated with an increased risk of obesity and weight gain, as shown in prospective studies and randomised controlled trials, respectively( Reference Te Morenga, Mallard and Mann 2 ). In another meta-analysis, a higher consumption of SSB was associated with an increased risk of type 2 diabetes( Reference Greenwood, Threapleton and Evans 3 ). However, available evidence in two meta-analyses suggests that a higher consumption of SSB is not associated with either pancreatic( Reference Gallus, Turati and Tavani 28 ) or colon cancer( Reference Zhang, Albanes and Beeson 29 ).
Less attention has been paid to the association between SSB consumption and cardiovascular risk, such as CHD or stroke. A recent meta-analysis of the association between SSB intake and the risk of CHD conducted by Huang et al. ( Reference Huang, Huang and Tian 30 ) included the same studies, and demonstrated the same strength of the association. However, that meta-analysis did not address whether SSB consumption is associated with the risk of hypertension and stroke. For hypertension, Dhingra et al. ( Reference Dhingra, Sullivan and Jacques 4 ) reported that consumption of ≥ 1 serving of soft drink per d was not significantly associated with an increased risk of higher blood pressure (BP; RR 1·18, 95 % CI 0·96, 1·44). However, in a subsequent study, Cohen et al. ( Reference Cohen, Curhan and Forman 6 ) found that participants who consumed ≥ 1 serving/d had an adjusted HR for incident hypertension of 1·13 (95 % CI 1·09, 1·17) compared with those who did not consume SSB. Furthermore, the PREMIER Study (an 18-month behavioural intervention trial) conducted by Chen et al. ( Reference Chen, Caballero and Mitchell 31 ) has suggested that after controlling for potential confounders, each 1 serving/d reduction in SSB consumption was associated with a 1·8 mmHg (95 % CI 1·2, 2·4) reduction in systolic BP and 1·1 mmHg (95 % CI 0·7, 1·4) reduction in diastolic BP. This association did not substantially change after further adjustment for weight change over the same period. The different findings on the association between SSB intake and the risk of hypertension, CHD and stroke might be due to biological mechanisms or to other reasons (study population, design, sample size or other factors). Based on the present meta-analysis of prospective studies, each additional 1 serving/d increase in SSB consumption was associated with a 8 and 17 % relative increase in the risk of incident hypertension and CHD, respectively. In the present meta-analysis, we did not find any significant association between SSB consumption and the risk of incident stroke, although a significant association was found in studies conducted in Caucasians. Currently, convincing reasons for the absence of any significant association in the total population but finding an association only in some ethnic subgroups are unknown. It is possible that genetic factors may play some role in the association of SSB intake with the risk of stroke.
The association between artificially sweetened beverage (ASB) intake and the risk of CVD has already been reviewed by Pereira( Reference Pereira 32 ) who reported that ASB intake may increase the risk of CVD; however, there could be a reverse causality bias because obese individuals may tend to preferentially consume ASB in order to reduce their weight gain and are also more likely to develop CVD. Furthermore, evidence from experimental studies supports that replacing SSB with ASB may be beneficial to decrease the risk of obesity.
Mechanisms
The effect of BMI and total energy intake could not fully explain the positive association between SSB consumption and the risk of incident hypertension or CHD, since both potential mediators have been controlled for in the majority of the included studies. Fructose, one of the major sweeteners in SSB, has been suggested to result in acute and chronic elevations of serum uric acid concentration, thereby leading to the activation of the renin–angiotensin system and, consequently, acute endothelial dysfunction, renal microvascular alteration and chronic Na retention( Reference Feig, Kang and Johnson 33 ). This mechanism may explain why SSB consumption could increase the risk of incident hypertension. With respect to the observed association between SSB consumption and the risk of CHD, fructose has been found to increase the levels of several circulating inflammatory factors, such as C-reactive protein, IL-6, TNF receptor 1 and TNF receptor superfamily, which are known to influence atherosclerosis, plaque stability and thrombosis, and are key factors in the pathogenesis of CVD( Reference Hotamisligil 34 ). Fructose has also been associated with increased insulin resistance, reduced HDL-cholesterol, higher visceral fat stores and higher TAG concentrations, as well as with reduced endothelial NO production. All these changes have been associated with a higher risk of CHD( Reference Duffey, Gordon-Larsen and Steffen 5 , Reference Fung, Malik and Rexrode 8 ).
Strengths and limitations
The strengths of the present study included the prospective study design, the large sample size, the long duration of follow-up and adjustment for many dietary and lifestyle factors in the included studies. However, several limitations should be considered. First, because of the observational nature of these studies, the possibility of residual and unmeasured confounding may have influenced the results. Second, errors may exist in measuring SSB consumption using FFQ since accuracy is dependent on an individual's memory and reporting. However, misclassification is usually non-differential and, thus, more likely to lead to an underestimation of the association. Third, all the studies used SSB consumption at baseline as exposure, and it is possible that participants may have changed their beverage habits during the follow-up period. Fourth, the majority of the included participants were white Americans. This limits the generalisability of our findings to other ethnic populations. Fifth, only several prospective studies were included for each outcome; however, the sample sizes of total incident cases were large enough (n>6000) and sufficient statistical power was available. Sixth, different criteria were used to define the outcomes. For hypertension, some studies used self-reported hypertension( Reference Cohen, Curhan and Forman 6 ), whereas others used systolic BP/diastolic BP ≥ 130/85 mmHg( Reference Dhingra, Sullivan and Jacques 4 , Reference Duffey, Gordon-Larsen and Steffen 5 , Reference Barrio-Lopez, Martinez-Gonzalez and Fernandez-Montero 7 ). However, the significant association remained in each subgroup (RR 1·09, 95 % CI 1·06, 1·13 for studies using self-reported hypertension; RR 1·04, 95 % CI 1·01, 1·08 for studies using systolic BP/diastolic BP ≥ 130/85 mmHg). With respect to the endpoints of CHD and stroke, the definitions were similar for each outcome (mostly via medical records). Seventh, dietary assessment errors (e.g. FFQ) affect the accuracy of the estimated ‘doses’. Additionally, different confounders were adjusted for in each study, and this may have affected the accuracy of our estimates in the dose–response meta-analysis. Eighth, the present results might have been potentially underestimated, as the included studies had adjusted for variables such as BMI, history of diabetes and hypertension in the models. Those variables could be in the causal pathway for the association between SSB intake and the risk of CHD/stroke. Ninth, although meta-regression was used to explore the potential sources of heterogeneity for the outcome of hypertension, none of the known variables was identified as a specific source of heterogeneity. Thus, caution should be exercised while interpreting pooled estimates showing large heterogeneity. Tenth, it is unclear whether the association that we have found is due to the sugar content of SSB or to related lifestyle factors associated with the consumption of SSB, such as other dietary practices, sedentary behaviours or lack of physical activity. However, the vast majority of the included studies have controlled for these dietary and lifestyle factors in the models. Nevertheless, further randomised controlled trials are warranted to confirm the observed association.
In conclusion, a higher consumption of SSB was associated with a higher risk of hypertension and CHD, but not with the risk of stroke. Notably, recent evidence suggests that increasing water intake in place of SSB was associated with a lower risk of weight gain( Reference Pan, Malik and Hao 35 ) and type 2 diabetes( Reference Pan, Malik and Schulze 36 ). The present results support recommendations to reduce the consumption of SSB in order to prevent and control CVD, although further large prospective studies or randomised controlled trials are warranted to confirm the observed association.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514004383
Acknowledgements
The present study was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2012HL26). The funder had no role in the design and analysis of the data or in the writing of this article.
The authors' contributions are as follows: B. X. designed the research; B. X. and D. Z. searched the databases and checked them according to the eligible criteria and exclusion criteria; M. T. B.-L., M. A. M.-G. and R. Z. acquired the data; B. X. and Y. H. analysed the data; B. X. wrote the draft of the paper; B. X., Y. H., K. H. R., S. L., R. Z., M. T. B.-L., M. A. M.-G. and D. Z. revised the paper; D. Z. was responsible for the final content. All the authors read and approved the final manuscript.
None of the authors has any conflict of interest to declare.







