The growing burden of obesity-associated chronic diseases is now one of the more important health issues in the world. Obesity and excess abdominal fat are risk factors for a broad range of chronic diseases such as hypertension, CVD and type 2 diabetes mellitus, all of which are now the principal causes of mortality worldwide(Reference Cutles, Glaeser and Shapiro1–Reference Waring, Roberts and Parker3). The burden of this complex of diseases is now becoming an acute problem in industrialising countries passing through an epidemiological and nutrition transition. Mexico, for example, is one of the countries with the highest prevalence of obesity, as found in a 2006 National Nutrition Survey of Mexico (Encuesta Nacional de Salud y Nutrición; ENSANUT 2006), and this prevalence is increasing year after year(Reference Olaiz-Fernández, Rivera-Dommarco and Shamah-Levy4).
Obesity has been described as a form of low-grade inflammation(Reference Maffei, Funicello and Vottari5). Inflammatory cytokines such as TNF-α, IL-1 and IL-6, which are important risk factors for chronic diseases, are elevated in obese individuals. These cytokines are principally produced through signalling pathways located within the adipocytes that make up the white adipose tissue. Their circulating concentrations directly correlate with adipocyte size and number(Reference Rodríguez-Rodríguez, Perea and López-Sobaler6). Leptin, an adipokine that promotes the sensation of satiety, plays an important role in promoting the expression of such inflammatory cytokines(Reference Stofkova7). Also, leptin is involved in the activation and infiltration of macrophages into white adipose tissue(Reference Rodríguez-Rodríguez, Perea and López-Sobaler6), the activation of natural killer cells(Reference La Cava and Matarese8), and suppresses the proliferation of CD4+ and CD25+ Treg cells(Reference De Rosa, Procaccini and Cali9), and also regulates the T-helper (Th) 1 and Th2 response(Reference Lord, Matarese and Howard10).
Micronutrient status is associated with obesity and may have an important effect on the inflammatory response(Reference García, Long and Rosado11). Studies have shown that low Zn serum concentration is associated with an increased risk of being obese(Reference García, Long and Rosado11). Zn may regulate the inflammatory response by up-regulating leptin synthesis, by directly activating monocytes, or through its role in Zn-α2-glycoprotein (ZAG) which is involved in TNF-α transcription(Reference Cristos, Mantzoros and Ananda12, Reference Gomez-Garcia, Hernandez-Salazar and Gonzalez-Ortiz13). A vitamin A-deficient diet in animal models is associated with increased leptin expression in white adipose tissue and brown adipose tissue(Reference Kumar and Scarpace14, Reference Bonet, Oliver and Pico15). Vitamin A and its active metabolites inhibit the inflammatory Th1 response and promote the Th2 response(Reference Dawson, Solana and Beal16). Vitamin E has also been found to function as an anti-inflammatory agent in studies concerned with inflammation and pulmonary function in asthma patients as well as inflammation in rats with induced asthma(Reference Tabak, Smit and Rasanen17, Reference Berdnikovs, Abdala-Valencia and McCary18). Vitamin C has been shown to have anti-inflammatory effects by reducing C-reactive protein (CRP) serum concentrations(Reference Wannamethee, Lowe and Rumley19, Reference Block, Jensen and Dietrich20). It is still not clear what effect each micronutrient has on these inflammatory response pathways among individuals who differ in adiposity and body mass. Also, studies have shown that the intake of certain foods, such as legumes, whole grains, fruits and vegetables, that have a high content of micronutrients including Zn, and vitamins A, C and E, have been associated with lower markers of inflammation(Reference Esmaillzadeh and Azadbakht21–Reference Salas-Salvado, Casas-Agustench and Murphy23).
The objective of the present study was to examine how micronutrient blood concentrations and measures of adiposity are associated with the inflammatory response among Mexican women with a high prevalence of overweight and obesity. The further clarification of this relationship could lead to the development of new preventive and therapeutic micronutrient interventions that contribute to the reduction in the burden of obesity and its complications.
Experimental methods
Study population
The population for this study was 280 women, 25–55 years of age, who resided in six rural communities located within the Mexican State of Querétaro. Women were excluded from the study if they had uncontrolled diabetes, if they had received treatment for obesity or had taken vitamin and mineral supplements in the previous 3 months. Pregnant and lactating women, as well as women who had any type of eating disorder, were also excluded from the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Human Research Committee of the School of Natural Sciences at the Universidad Autónoma de Querétaro (UAQ). Written informed consent was obtained from all subjects/patients.
The sample size of 280 subjects in this study was intended to find a significant OR of 0·50 or 1·75 between two independent groups with different concentrations of cytokines, with an α error of 5 %, β error of 20 %, and an estimated prevalence of a micronutrient deficiency of 20 %(Reference Dupont and Plummer24).
Study design
This study was cross-sectional in design, in which all enrolled women were first interviewed during an initial household visit to determine clinical histories of chronic disease. Subsequently, these women were then transported at different dates to the Metabolic Unit at the School of Natural Sciences within UAQ, where anthropometric and body composition determinations were made in one session.
A single, basal 5 ml blood sample was drawn from all women who had been fasting for the previous 12 h for the determination of glucose concentrations, lipid profile, Zn, vitamins A, C and E concentrations and concentrations of inflammatory and adaptive immune response cytokines. These samples were collected from the women, at the local health clinic in their respective communities.
Anthropometry and body composition methods
The women wearing light clothing were weighed using a scale (SECA ONDA Mod 843; SECA) with a precision of 1·0 g, while their stature was determined with a precision of 0·1 cm using a portable stadiometer (SECA Mod 206; SECA). Hip circumference was determined with a precision of 0·1 cm using flexible metal tapes (SECA Mod 200; SECA). All measurements were done, non-consecutively, in duplicate by personnel, that had been previously standardised in their application according to procedures developed by Lohman et al. (Reference Lohman, Roche and Martorell25). Women were then categorised as normal weight (BMI < 24·9 kg/m2), overweight (BMI between 25·0 and 29·9 kg/m2) and obese (BMI >30·0 kg/m2), using the WHO classification system(26, Reference James, Leach and Kalamara27).
Body composition determinations, percentage of body fat and percentage of abdominal fat, were carried out during this session using DEXA (Hologic, Mod Explorer) in the Metabolic Unit of UAQ, by a certified technician. High body fat was considered with values ≥ 30 % of total body mass(28). Abdominal fat was obtained as described previously(Reference Hill, LaForgia and Coates29). Briefly, abdominal fat is determined from a 10 cm rectangle that is drawn in the X-ray body diagram, immediately above the ileac crest of the subject and it is extended to the sides over the border of the subject's anatomy.
Laboratory methods
Leptin and C-reactive protein determinations
Concentrations of leptin and CRP in plasma were measured in duplicate using commercial ELISA kits (Human Leptin Elisa Kit, Linco Research; High Sensitivity C-Reactive Protein Elisa Kit, Bioquant).
Determination of inflammatory and adaptive immune response cytokines
The concentrations of the inflammatory-related cytokines, IL-1β, IL-6, IL-8, TNF and IL-12p70, and the anti-inflammatory cytokine IL-10 were measured simultaneously using flow cytometry analysis with the human inflammation kit (Becton Dickinson). Briefly, the serum samples (50 μl) were incubated 1·5 h in the dark with the appropriate dilution of the capture beads, which were then washed by centrifugation and followed by their incubation for an additional 1·5 h. The beads were then washed again by centrifugation and quantified in a FACSCAN (Becton Dickinson) equipped with a 488 nm laser. The data were analysed using the BD CellQuest software, with positive controls provided by the supplier.
Determination of zinc in plasma
Zn concentrations in plasma were measured using flame atomic absorption spectrophotometry (Perkin Elmer, Mod. Analyst 700) using the corresponding certified standards. Zn deficiency was defined as plasma concentrations of < 700 μg/l(Reference Hess, Peerson and King30).
Determination of vitamins A, E and C in plasma
Vitamins A and E were simultaneously determined by reversed-phase HPLC (WATERS) using a C18 column (WATERS) with a mobile phase of 100 % methanol (J. T. Baker) and a 1 ml/min flow with a temperature of 40°C, measured at a wavelength of 300 nm. Vitamin C was measured by HPLC (WATERS), using a C18 column (WATERS) with a mobile phase of 0·01 m-NaH2PO4 and 0·2 mm-EDTA, a 0·85 ml/min flow, and measured at a wavelength of 254 nm.
Determination of glucose and lipid profile
Fasting glucose concentration was measured by an enzymatic-colorimetric method using the commercial kit Glucose-PAP (Elitech) with a Clinical Analyser Bayer RA-50 (Bayer Diagnostics). Plasma concentration of total cholesterol, HDL and total TAG were measured using commercial kits (Cholesterol SL, Triglycerides, Cholesterol HDL SL 2G, Elitech) in a Bayer RA-50 Analyzer (Bayer Diagnostics). LDL was calculated from the previously mentioned data using the Friedewald method(Reference Friesdwald, Levy and Fredrickson31).
Biochemical and immunological assays of all samples were carried out in duplicate in the Human Nutrition Laboratory in the School of Natural Sciences at UAQ.
Data analysis
Data were entered in Excel (Microsoft), verified, and checked for range and consistency. Women were considered Zn-deficient if they had plasma concentrations below 700 μg/l(Reference Hess, Peerson and King30), and vitamin A-deficient if they had plasma concentrations < 200 μg/l(Reference Gibson32). Women were classified as vitamin E-deficient if they had concentrations < 5 μg/ml or < 2·4 μmol α-tocopherol:mmol lipid, and finally, vitamin C-deficient if they had plasma concentrations < 2 μg/ml(Reference Gibson32, 33). Women were classified as having high glucose if they had a fasting plasma concentration of glucose above 1260 mg/l(34). Women were classified with normal lipid concentration if they had cholesterol < 2000 mg/l, TAG < 1500 mg/l and HDL-cholesterol >350 mg/l(35).
The primary end points for this analysis were the plasma concentrations of pro- and anti-inflammatory cytokines and chemokines among all enrolled women from whom blood samples were taken. Means and standard deviations of physical and biochemical characteristics of enrolled women were first determined to summarise the data.
Associations between measures of obesity, total body fat in kg and percentage, and abdominal fat and plasma concentrations of the pro-inflammatory cytokines IL-1, IL-6, IL-8, IL-12, TNF-α and the anti-inflammatory cytokine IL-10 cytokine were determined as a first step in the overall analysis. Conventional analytical techniques could not be used to analyse these cytokines as endpoints, since an important proportion of samples had no detectable concentration of cytokines. Accordingly, ordered logistic regression analyses were used, which models the probability distributions of cytokine values categorised into three levels ordered from lowest to highest: undetectable, < median of positives, >median of positives(Reference Agresti36). The inclusion of BMI, abdominal fat (%) and waist-to-height index in the model tested the hypothesis that the probability distributions of categorised cytokine values will differ according to differences in these measures expressed as an OR. Ordinal logistical analyses were then carried out to determine the associations between leptin with the pro-inflammatory and anti-inflammatory cytokines. Leptin analysis was controlled for age and CRP concentrations. These analyses were meant to determine whether leptin is the intermediate mechanism underlying the associations between measures of obesity and body fat and cytokine plasma concentrations.
A final set of analyses was then carried out to test the hypothesis that the probability distributions of categorised cytokine values will vary by differences in the micronutrient plasma concentration again expressed as an OR. As such, analyses were first carried out with the variables for plasma concentrations of vitamin A, vitamin C, vitamin E adjusted for lipids, and Zn included separately in models for each cytokine. Models were then run, which included all the micronutrient variables together in models for each cytokine to control for any interactions between the different micronutrients analysed. Thus, results are presented adjusted for micronutrient concentrations all together, age and CRP concentrations, and further adjusted for BMI. Statistical significance was set at a probability level of < 0·05 and < 0·1 for interactions. Data were analysed using ordered logistics procedure (OLOGIT) in the STATA (version 10) software (STATACorp LP).
Results
A total of 280 women were enrolled in the study. The anthropometric and metabolic characteristics of the women at the beginning of the study are summarised in Table 1. The prevalence of obesity and overweight was 43·9 and 36·4 %, respectively. In addition, 21·7 % had hypertriacylglycerolaemia, 37·8 % had high total cholesterol and 11·8 % of the women had low levels of HDL. Overall, 4, 15·5 and 16·7 % of women had deficiencies of vitamin C, vitamin E and Zn, respectively, while no women were found to have vitamin A deficiency. When adjusting for total lipids, 11 % of the participants had vitamin E deficiency.
Table 1 Physical and biochemical characteristics of the subjects (Mean values and standard deviations)

CRP, C-reactive protein.
a,b,c Mean values within a row with unlike superscript letters were significantly different (Tukey test, P< 0·05).
Ordered logistical regression models were first used to analyse the associations between measures of obesity and adiposity with probability of higher cytokine and chemokine levels. Women with higher BMI, a greater percentage of abdominal fat or a greater waist-to-height index were found to have an increased probability of having higher concentrations of IL-6 (OR 1·05, 95 % CI 1·01, 1·09, P= 0·01; OR 1·03, 95 % CI 1·00, 1·07, P= 0·05 and OR 12·89, 95 % CI 0·92, 179·5, P= 0·04, respectively). No significant association was found between increased odds for the other cytokines and other anthropometric measures of obesity (Table 2). No association was found between leptin and cytokines when included in the models.
Table 2 Associations between body composition measures and serum innate chemokine and cytokines (n 280) (Odds ratios and 95 % confidence intervals)
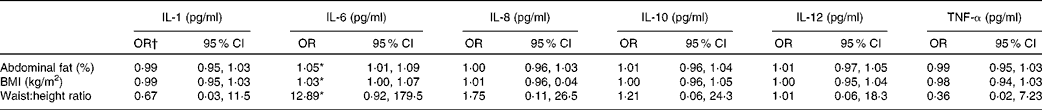
* P< 0·05.
† OR represents the odds that chemokine or cytokine levels (categorised into three levels: non-detectable, < median, >median) will be greater among women with greater fat and anthropometry measures. Micronutrient concentrations were included in the model along with C-reactive protein concentrations and age of women.
The ordered logistical regression models were then used to analyse the associations between serum micronutrient concentrations and the probability of higher cytokine and chemokine concentrations. The OR of having higher concentrations of TNF-α, IL-6, IL-10 and IL-12 were reduced for women with higher concentrations of plasma Zn (OR 0·63, 95 % CI 0·42, 0·96, P= 0·03; OR 0·57, 95 % CI 0·39, 0·86, P= 0·025; OR 0·63, 95 % CI 0·41, 0·96, P= 0·04 and OR 0·62, 95 % CI 0·41, 0·95, P= 0·03, respectively) (Table 3). Higher concentrations of vitamin A were associated with reduced risks of higher levels of IL-1 and IL-12 (OR 0·97, 95 % CI 0·95, 0·99, P= 0·03; OR 0·97, 95 % CI 0·94, 0·99, P= 0·03, respectively). This association disappears when adjusting for BMI. No associations between inflammation markers and vitamin C or vitamin E adjusted for lipids were found.
Table 3 Associations between micronutrient status and serum innate chemokine and cytokine levels (n 280) (Odds ratios and 95 % confidence intervals)

* P< 0·05.
† OR represents the odds that chemokine or cytokine levels (categorised into three levels: non-detectable, < median, >median) will be greater among women with greater serum micronutrient concentrations.
‡ Micronutrient concentrations were included in the model along with C-reactive protein concentrations and age of women.
§ As in model I and further adjusted for BMI.
Discussion
The present study has characterised the relationship between inflammatory cytokine markers and measures of obesity and adiposity among women, and also how serum concentrations of micronutrients are associated with inflammatory cytokine markers. Higher concentrations of plasma Zn were associated with reduced levels of inflammatory cytokines.
The significant association found between IL-6 and measures of obesity and body composition is consistent with other studies which have found that this cytokine is associated with adiposity(Reference Fried, Bunkin and Greenberg37). Mohamed-Ali et al. (Reference Mohamed-Ali, Goodrick and Rawesh38), for example, reported that approximately 30 % of in vivo circulating levels of IL-6 was secreted by adipose tissue and that this release was greater in obese subjects. Vozarova et al. (Reference Vozarova, Weyer and Hanson39) also found that fasting plasma IL-6 concentrations were positively related to adiposity but negatively related to insulin action in Pima Indians. In contrast to our findings, TNF-α also has been reported to be significantly elevated in obese, insulin-resistant rodents and humans(Reference Kern, Saghizadeh and Ong40, Reference Kern, Ranganathan and Li41). However, Kern et al. (Reference Kern, Ranganathan and Li41), reported that IL-6 levels were much higher in both adipose tissue and plasma in obese subjects while no significant differences in TNF-α secretion by adipose tissue depots was found between obese and lean subjects. These inconsistent observations support the lack of association for this cytokine and adiposity measures found in the present study.
The lack of correlation between leptin levels with any of the inflammation cytokine markers is not consistent with reports from other studies(Reference Lord, Matarese and Howard42). However, the relationship between leptin and inflammatory cytokines reported in previous studies is not always consistent. For example, an inverse relationship between leptin and IL-6 as well as other inflammation markers was found among rheumatoid arthritis patients(Reference Popa, Netea and Radstake43). In vitro studies of human and rat monocytes have also found that leptin enhances the expression of pro-inflammatory cytokines like IL-6, IL-8 and TNF-α(Reference Lofreda, Yang and Lin44, Reference Zarkesh-Esfahani, Pockley and Metcalfe45). Xiao et al. (Reference Xiao, Xia-Zhang and Vulliemoz46), in contrast, reported a reduction in IL-6 and TNF-α among primates immunised with endotoxin after treating them with leptin. The lack of an association between leptin and inflammation found in this study may be due to the low grade of inflammation and cytokine concentrations seen in obesity, which may not provide adequate statistical power relative to that reported in acute inflammation states or in cell lines. Overall, these findings suggest that, in this population, leptin may not be directly involved in the pathway that leads to chronic inflammation among obese individuals.
The reduced odds of higher levels of the inflammatory markers TNF-α, IL-6 and IL-12 among women with higher Zn concentration is similar to the findings by Bao et al. (Reference Bao, Prasad and Beck47). They reported that Zn supplementation of elder subjects was associated with decreased concentrations of plasma inflammatory cytokines. Much research has been carried out about Zn concerning the pathways that may be involved in the association of Zn and the immune system; there is still no consensus. However, the strongest theory involves Zn regulation of Zn-dependent enzymes related with the immune system(Reference Mracek, Gao and Tzanavari48). One of these enzymes is ZAG, a soluble cytokine that down-regulates the expression of TNF-α. Bao et al. (Reference Bao, Bing and Hunter49) consider ZAG an important beneficial factor for obesity(Reference Bao, Bing and Hunter49). The expression of ZAG is down-regulated in obese subjects(Reference Marredes, Martinez and Moreno50). Thus, Zn may protect against chronic inflammation through the expression of ZAG and its down-regulation effect on TNF-α.
In the present study, the odds of having higher levels of the anti-inflammatory cytokine IL-10 was also reduced with high Zn concentrations. Similarly, it has been observed in both in vitro and human studies that Zn supplementation decreases IL-10 concentrations(Reference Metz, Schroder and Overbeck51, Reference Sandstead, Prasad and Penland52). In addition, obese individuals have increased pro-inflammatory and anti-inflammatory cytokines(Reference Fain53). Obese women, for example, before a gastric bypass, had significantly higher IL-10 concentrations compared to normal-weight women(Reference Dalmas, Rouault and Abdennour54). TNF-α, IL-6 and IL-10 concentrations were significantly higher in obese patients with heart failure than in non-obese patients with mild heart failure(Reference Hamdy55). This has also been observed in patients during an infectious disease episode, where the concentrations of the anti-inflammatory cytokine IL-10 are increased in response to increased inflammatory cytokine concentrations in order to minimise damage to the host and to avoid tissue damage. This simultaneous response then allows the immune response to return to homeostasis once the infection or the antigen is cleared(Reference Sanjabi, Zenewicz and Kamanaka56). Something similar might be occurring in obese or overweight individuals, or with Zn deficiency, where elevated concentrations of IL-10 may be part of a mechanism to counteract the effect of the pro-inflammatory cytokines(Reference Fain53). The anti-inflammatory effect of IL-10 has been attributed to its ability to inhibit the maturation of antigen-presenting cells, but preserving the capacity of the cells to load the antigens. Also, it has been suggested that it may produce a pro-inflammatory response when antigen-loaded antigen-presenting cells activate local immune responses(Reference Mocellin, Panelli and Wang57). Thus, Zn concentrations may play an important role in the pleiotropic effects of IL-10.
Vitamin A is recognised to down-regulate the expression of inflammation markers and promote a Th2 immune response by binding with its membrane receptor retinoic acid receptor-α that inhibits the production of interferon-γ, a mediator of other inflammation markers(Reference Dawson, Solana and Beal16, Reference Kumar, Sunvold and Scarpace58). In this study, higher concentrations of vitamin A are associated with decreased odds of having higher levels of inflammation markers, although this association is only found for the cytokines IL-1 and IL-12 and is not as great as that seen with Zn. When adjusting for obesity, no effect was found between vitamin A concentrations and the levels of inflammation markers. These results may reflect the fact that subjects in this study were not vitamin A-deficient. In vitamin A deficiency, there is an increase of pro-inflammatory cytokines that promotes a Th1-type inflammatory response, while the production of anti-inflammatory cytokines, such as IL-10, is reduced(Reference Long and Santos59, Reference Aukrust, Müller and Ueland60). For example, in Indonesian children, vitamin A deficiency was associated with a higher Th1 response(Reference Wieringa, Dijkhuizen and West61). Similarly, the production of IL-10 was negatively associated with vitamin A stores in adult men(Reference Ahmad, Haskell and Raqib62). Thus, vitamin A deficiency increases the production of pro-inflammatory cytokines and reduces the concentration of anti-inflammatory cytokines.
Vitamin C and vitamin E:lipids were not associated with any of the cytokines studied. Findings on the relationship between vitamin C and vitamin E with inflammatory markers have been inconsistent(Reference Berdnikovs, Abdala-Valencia and McCary18–Reference Block, Jensen and Dietrich20, Reference Beyer63–Reference Bruunsgaard, Poulsen and Pedersen66). For example, intakes of vitamin C and vitamin E were not associated with CRP or IL-6 in 5181 participants from the Multi-Ethnic Study of Atherosclerosis(Reference De Oliveira Otto, Alonso and Lee64). In contrast, Mah et al. (Reference Mah, Matos and Kawiecki65) found an inverse relationship between vitamin C status and inflammatory markers among obese men compared with a group of lean adult men in a cross-sectional study, but found no association between vitamin E status and CRP concentrations or inflammatory cytokines. Similarly, Wannamethee et al. (Reference Wannamethee, Lowe and Rumley19) reported that vitamin C status was inversely associated with CRP concentrations and lower endothelial dysfunction in men with no history of CVD or diabetes. These epidemiological studies show that vitamin C status is associated with CRP while no associations have been found between vitamin C status and vitamin E status with the inflammatory cytokine response, similar to the findings in the present study.
Supplementation studies have also reported mixed results. Bruunsgaard et al. (Reference Bruunsgaard, Poulsen and Pedersen66) found no difference in the concentration of IL-6 or TNF-α among adult men supplemented with vitamins C and E for 36 months compared to men who received a placebo. In contrast, Block et al. (Reference Block, Jensen and Dietrich20) reported that vitamin C supplementation for 2 months was associated with a 24 % reduction in plasma concentrations of the inflammatory marker CRP. Increases in vitamin E plasma concentrations after supplementing with vitamin E and vitamin C for 2 weeks were associated with lower TNF-α concentrations in adult men with impaired fasting glucose(Reference Rizzo, Abbatecola and Barbieri67). These inconsistencies regarding the effect of vitamin E on inflammation may be due to the different forms of this vitamin that are found in the diet or given as supplements. Berdnikov et al. (Reference Berdnikovs, Abdala-Valencia and McCary18), for example, reported that the α-tocopherol and d-α-tocopherol isoforms of vitamin E have opposing immunoregulatory effects on the inflammatory response. The lack of consistency in the clinical trials may also be explained by the differences in the supplementation period that range from 2 weeks to 3 years and/or the doses given. More studies are needed to understand the effect of vitamin C and vitamin E on inflammatory markers.
A number of methodological limitations of our study need to be addressed. The cross-sectional design of the study complicates efforts to determine causality between body composition and obesity, serum micronutrient concentrations and inflammatory cytokines. For example, it is not possible to determine whether elevated inflammatory cytokines may be affecting serum micronutrient concentrations or whether micronutrient concentrations are affecting inflammatory cytokine levels. The effects of inflammation on micronutrient concentrations were partly controlled by including CRP in all the models. There were no significant differences in the associations between models that included CPR compared to models that did not include this inflammatory marker, suggesting that micronutrient concentrations could be affecting inflammatory cytokine levels.
Associations were found between specific micronutrient serum concentrations and the odds of having higher concentration of inflammatory markers. The findings in the present study suggest that micronutrients play a different role in low-grade chronic inflammation among individuals with differing degrees of adiposity and obesity. Overall, it was found that higher Zn concentration is associated with reduced risks of higher concentration of inflammation markers. These results are preliminary and so must be interpreted with caution. It is important to continue addressing the relationship between these micronutrients and the inflammatory response especially among populations that differ in the prevalence of overweight and obesity. Such efforts will determine whether such relationships change with changing adiposity, and so establish how specific nutrients may be interacting with adipose tissue to regulate the production of inflammatory cytokines and so modify low-grade inflammation occurring in obesity.
Acknowledgements
The authors wish to acknowledge the work of the nutritionists who participated in the fieldwork, Rosa María Rodríguez and Shadia Elian, and also the health clinics in the communities where the study took place. The authors declare no conflicts of interest. The present study was partially funded by CONACYT Project no. SEP-2004-C01-48183. The authors' responsibilities were as follows: G. Z. participated in the data analysis and interpretation, and wrote the initial draft of the manuscript. K. Z. L. contributed to the study design, data analysis and interpretation. O. P. G. contributed with the concept and study design, study implementation, and contributed to the writing of the manuscript. M. d. C. C. contributed in the writing of the manuscript. T. A. assisted in the data management and interpretation. L. M. S. contributed with the analysis of the cytokines. J. L. R. contributed to the concept and study design and provided important intellectual content to the manuscript.





