Many previous epidemiological studies( Reference Zhang, Ho and Chen 1 – Reference Gaudet, Britton and Kabat 3 ) have shown a protective role of fruit and vegetables in breast cancer. The possible protective compounds in fruit and vegetables may include carotenoids. Carotenoids are lipid-soluble pigments present in red, yellow, orange and dark-green fruit and vegetables. The main carotenoids are α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein and zeaxanthin( Reference Mangels, Holden and Beecher 4 ).
There are several mechanisms to be proposed in which specific carotenoids may act as a protective factor against breast cancer. Besides their overall influence on the enhancement of immune function, the protection of cells against DNA damage, the stimulation of gap junctional intercellular communication, the induction of detoxifying enzymes and the inhibition of cellular proliferation, it has been shown that they also have specific activities. α-Carotene may suppress cytochrome P450 1A1, an activator of procarcinogens( Reference Cooper, Eldridge and Peters 5 ). β-Carotene may control growth-inhibitory and pro-apoptotic effects in cancer cells through the redox regulation of the nuclear transcription factor NF-κB( Reference Hirsch, Atzmon and Danilenko 6 ). β-Cryptoxanthin may stimulate the expression of RB, an anti-oncogene, and p73, a p53-related gene( Reference Nishino, Tokuda and Murakoshi 7 ); lycopene is the most efficient singlet oxygen quencher in vitro ( Reference Tsen, Tsen and Kiang 8 ), whereas lutein and zeaxanthin are scavengers of radical oxygen species( Reference Bohm, Tinkler and Truscott 9 ).
Fruit and vegetables differ in their nutrient content as groups. To account for huge differences in the nutrient content of the variety of fruit and vegetables, it is worthwhile to perform studies at the nutrient level, i.e. to test for the association between dietary carotenoid intakes (derived from food consumption data) and the risk of breast cancer. However, the findings from epidemiological studies on carotenoid intakes in relation to the risk of breast cancer have been inconclusive. Some case–control studies( Reference Levi, Pasche and Lucchini 10 – Reference Bohlke, Spiegelman and Trichopoulou 20 ) and cohort studies( Reference Cui, Shikany and Liu 21 – Reference Cho, Spiegelman and Hunter 26 ) have noted an inverse association of breast cancer with specific carotenoid intakes, whereas others have not( Reference Terry, Jain and Miller 27 – Reference Wang, Baumgartner and Yang 30 ). A few studies( Reference Mignone, Giovannucci and Newcomb 13 , Reference Larsson, Bergkvist and Wolk 23 , Reference Cho, Spiegelman and Hunter 26 ) have suggested that the protective effect of specific carotenoid intake on the risk of breast cancer was mainly confined to smokers. The association of β-carotene intake with the risk of breast cancer was stronger among women who consumed 15 g or more of alcohol per d( Reference Zhang, Hunter and Forman 24 ). Moreover, some studies( Reference Cui, Shikany and Liu 21 , Reference Larsson, Bergkvist and Wolk 23 ) have reported that the associations between carotenoid intakes and the risk of breast cancer are stratified by oestrogen receptor (ER) and progesterone receptor (PR) status. However, most of the aforementioned studies have been conducted in Western countries. More research in non-Western populations is needed to clarify this association.
Chinese populations generally have a high intake of carotenoid-rich vegetables and fruit( Reference Zhang, Ho and Chen 1 ). The rates of active cigarette smoking and alcohol consumption among Chinese women are very low. However, the passive smoking rate of female was high (43·6 %)( Reference Tao Xu 31 ). This provided a good opportunity to examine the relationship between carotenoid intake and the risk of breast cancer among non-smoking, non-alcohol drinking women. However, few studies have been conducted so far among Chinese women to examine this issue( Reference Huang, Zhang and Holman 12 ). Therefore, the purpose of the present study was to investigate the relationship between dietary carotenoid (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene) intake and the risk of breast cancer among Chinese women.
Materials and methods
Study subjects
This is an ongoing case–control study beginning in September 2011. The details of this study have been reported previously( Reference Zhang, Pan and Li 32 ). In brief, potential case subjects were recruited from patients admitted to the three teaching and general hospitals in the areas being studied. Inclusion criteria were female subjects aged 25–70 years old and natives of Guangdong province or having lived in Guangdong for at least 5 years, with histologically confirmed breast cancer diagnosed within 3 months before the interview. Women were excluded if they had a prior history of breast cancer or other cancers. During September 2011 to May 2013, a total of 561 (93·3 %) cases out of 601 eligible cases took part in the study. Control subjects were patients with no history of any cancers and admitted to the same hospitals during the same time period as the case subjects. They were frequency matched by age (5-year interval) and residence (rural/urban) to the case patients. They were selected from the Departments of Ophthalmology, Plastic and Reconstructive Surgery, Vascular Surgery and Ear–Nose–Throat. Totally, 561 (94·4 %) controls out of 594 eligible controls were successfully interviewed.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethical Committee of School of Public Health, Sun Yat-sen University. Written informed consent was obtained from all subjects.
Data collection
Data were collected by trained interviewers through a face-to-face interview based on a structured and previously validated questionnaire on sociodemographic data, current weight, height, menstrual and reproductive history, menopausal status, use of exogenous hormones, use of contraceptive drugs, family history of cancer, medical history, medication treatment, dietary habits, active and passive smoking, alcohol drinking and physical activities. BMI was calculated by dividing body weight (in kg) by height (in m) squared. Relevant medical diagnosis, histological findings, and ER and PR status were abstracted from the medical records. Regular smoking was defined as smoking at least one cigarette per d for more than six consecutive months. Exposure to second-hand smoke (SHS) was defined as non-smokers who reported being exposed to the smoke exhaled by smokers at least 15 min 1 d in a week. Regular drinking was defined as drinking alcohol at least once per week over the past year.
Dietary intake assessment
An interviewer-administered FFQ( Reference Zhang and Ho 33 ) consisting of eighty-one food items was used to collect information on habitual dietary intake during the previous year before diagnosis for breast cancer patients or before the time of interview for controls. The daily nutrient intakes of energy, α-carotene, β-carotene, lycopene, β-cryptoxanthin and lutein/zeaxanthin were calculated by summing the product of the frequency of consumption, the usual portion consumed and the nutrient content of each food item. The nutrient content values were derived from the Chinese Food Composition Table( Reference Yang 34 ) and the US Department of Agriculture nutrient database( Reference Exler 35 ). Food pictures with usual portion size were provided to help participants quantify the portions consumed.
The reproducibility and validity of the FFQ have been assessed previously( Reference Zhang and Ho 33 ). The correlation coefficients between two FFQ administered 1 year apart were 0·41 for α-carotene, 0·47 for β-carotene, 0·60 for β-cryptoxanthin, 0·51 for lycopene and 0·42 for lutein/zeaxanthin. The correlation coefficients for the FFQ and 18 d dietary records were 0·23 for α-carotene, 0·37 for β-carotene, 0·35 for β-cryptoxanthin, 0·30 for lycopene and 0·20 for lutein/zeaxanthin.
Statistical analysis
All statistical analyses were conducted using SPSS 13.0 software, and the significance level set at 0·05 for the two-tailed P value. Demographic characteristics and potential risk factors were compared between the cases and controls using the t test or Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical variables. Quartiles of specific carotenoids were defined based on the distribution of the controls. Unconditional logistic regression models were used to calculate OR and 95 % CI of each quartile using the lowest quartile group as the reference. The relationships between carotenoid intake and the risk of breast cancer were further examined after adjusting for various potential confounding factors using multivariate logistic regression models. Income, BMI (continuous), passive smoking (yes/no) and first-degree relative with cancer (yes/no) were selected as potential confounders based on the comparison of baseline characteristics between the cases and controls. Total energy intake was also adjusted using the residual method( Reference Willett, Howe and Kushi 36 ). Tests for trend were performed by entering the categorical variables as continuous variables in the models.
Breast cancer defined by ER and PR status appears to be aetiologically and clinically heterogeneous( Reference Althuis, Fergenbaum and Garcia-Closas 37 , Reference Ursin, Bernstein and Lord 38 ). The analysis stratified by ER and PR status was also performed to determine whether the association between carotenoid intake varied with ER and PR status. Because some risk factors for breast cancer have different associations among pre- and postmenopausal women( Reference Huang, Hankinson and Colditz 39 ), the relationships between specific carotenoid intake and the risk of breast cancer may differ by menopausal status. Therefore, stratified analysis by menopausal status (pre- v. postmenopausal) was conducted. Stratified analysis by passive smoking status was also conducted. The interactions between menopausal status, passive smoking and carotenoid intake on the risk of breast cancer were evaluated using a multiplicative model. The product term was included in the multivariate logistic regression to test for multiplicative interaction. Furthermore, sensitivity analysis was conducted by excluding women with smoking history and alcohol consumption.
Results
The sociodemographic and other characteristics of the study subjects are shown in Table 1. Compared with the controls, the cases were more likely to have a history of first-degree relative with cancer and a history of passive smoking from other persons, and were more likely to have a lower household income. No significant differences were observed between the cases and controls in educational level, marital status, BMI, use of oral contraceptive, alcohol consumption or reproductive factors, including age at menarche, age at menopause, nulliparous, number of live births, age at first live birth and months of breast-feeding.
Table 1 Sociodemographic and selected risk factors of breast cancer among breast cancer cases and controls (Mean values and standard deviations; number of subjects and percentages)

* Among women who have had a live birth.
† Among women who had breast-fed.
‡ Among menopausal women.
The mean intakes of main food groups and carotenoids are presented in Table 2. Compared with the controls, intakes of α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein/zeaxanthin, total energy, vegetables and white meat were significantly lower in the case subjects, whereas red meat intake was significantly higher in the case subjects.
Table 2 Consumption of specific carotenoids and selected dietary variables between breast cancer cases and controls (Mean values, and median values with their 25th–75th percentiles)
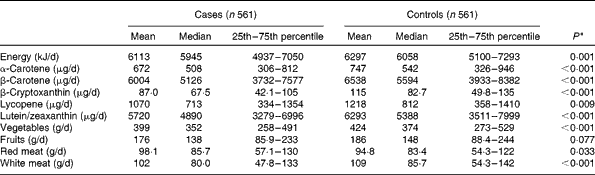
* Wilcoxon rank-sum test comparing the median consumption levels between the cases and controls.
The main food source of α-carotene was carrots. β-carotene was mainly from spinach and carrots. β-Cryptoxanthin was supplied by oranges and papaya, whereas lycopene was from watermelon and tomatoes. Lutein and zeaxanthin were derived primarily from spinach and broccoli.
The OR and corresponding 95 % CI for breast cancer risk according to specific carotenoid intakes are summarised in Table 3. After adjustment for income, passive smoking, first-degree relative with cancer and total energy intake, a significant inverse association was observed between dietary intakes of α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin and the risk of breast cancer. The multivariate OR for the highest quartile of intake compared with the lowest quartile of intake were 0·59 (95 % CI 0·42, 0·85) for α-carotene, 0·52 (95 % CI 0·36, 0·74) for β-carotene, 0·35 (95 % CI 0·25, 0·52) for β-cryptoxanthin and 0·48 (95 % CI 0·33, 0·69) for lutein/zeaxanthin. Further adjustment for other dietary factors including white meat and red meat intakes did not change the results appreciably. No significant association was found between lycopene intake and the risk of breast cancer, with the adjusted OR of 0·89 (95 % CI 0·61, 1·30).
Table 3 Risk for breast cancer according to quartiles of the daily intake of carotenoids (Odds ratios and 95 % confidence intervals)
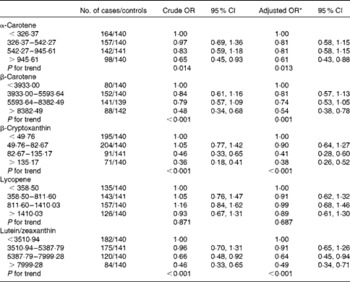
* Adjusted for income, second-hand smoke, first-degree relative with cancer, total energy intake, red meat intake and white meat intake.
Information on the ER and PR status of the tumour was available for 492 (87·7 %) of the cases. Of these breast cancer cases, the cases positive for ER and PR accounted for 383 (77·8 %) and 357 (72·5 %), respectively. Of the total 561 cases, 353 were ER-positive and PR-positive (ER+PR+), 105 were ER-negative and PR-negative (ER − PR − ), thirty were ER-positive and PR-negative (ER+PR − ), four were ER-negative and PR-positive (ER − PR+) and sixty-nine were undefined. ER − PR+ breast cancers were not analysed separately because of the small number of cases. An inverse association between dietary intakes of α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin and the risk of breast cancer was observed in all subtypes as defined by ER and/or PR status, although there was no statistical significance among women with ER+/PR − breast cancer tumours (Table 4).
Table 4 Risk for breast cancer according to quartiles of the daily intake of carotenoids stratified by oestrogen receptor (ER) and/or progesterone receptor (PR) status (Odds ratios and 95 % confidence intervals)
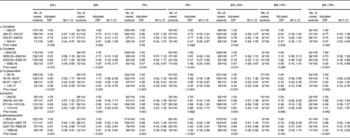
* Adjusted for income, passive smoking, first-degree relative with cancer, total energy intake, red meat intake and white meat intake.
Table 5 shows the association between carotenoid intake and the risk of breast cancer stratified by menopausal status. An inverse association between dietary intakes of α-carotene, β-carotene and lutein/zeaxanthin and the risk of breast cancer was only found in pre-menopausal but not in postmenopausal women. However, β-cryptoxanthin intake was observed to be inversely associated with the risk of breast cancer in both pre- and postmenopausal women. Lycopene intake was not related to the risk of breast cancer regardless of menopausal status.
Table 5 Risk for breast cancer according to quartiles (Q) of the daily intake of carotenoids stratified by menopausal status (Odds ratios and 95 % confidence intervals)

* Adjusted for income, passive smoking, first-degree relative with cancer, total energy intake, red meat intake and white meat intake.
Stratified analyses by passive smoking status were also conducted. Except for β-cryptoxanthin, an inverse association between dietary intakes of α-carotene, β-carotene and lutein/zeaxanthin and the risk of breast cancer was confined to non-smoking women who were exposed to SHS. No relationship was found between lycopene intake and the risk of breast cancer in all women or in specific type of women (Table 6).
Table 6 Risk for breast cancer according to quartiles (Q) of the daily intake of carotenoids stratified by passive smoking status (Odds ratios and 95 % confidence intervals)

* Adjusted for income, first-degree relative with cancer, total energy intake, red meat intake and white meat intake.
In the present study, there were eighteen women who were current or former smokers, and restricting the analysis to non-smokers yielded results similar to those of the overall group. Sensitivity analysis excluding women with alcohol consumption was also conducted, and the results showed no substantial change (data not shown).
Discussion
The present hospital-based case–control study evaluated the relationship between carotenoid intake and the risk of breast cancer among Chinese women. The results showed that consumption of α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin was inversely associated with the risk of breast cancer. The protective effect of specific carotenoid intake on the risk of breast cancer may be more pronounced among pre-menopausal women and women who were exposed to SHS; however, this inverse association did not differ by ER and PR subtypes of breast cancer status. No association was found between dietary lycopene intake and the risk of breast cancer.
The relationship between specific carotenoid intake and the risk of breast cancer has been examined in many epidemiological studies( Reference Gaudet, Britton and Kabat 3 Reference Huang, Zhang and Holman 12 Reference Mignone, Giovannucci and Newcomb 13 Reference Cui, Shikany and Liu 21 – Reference Terry, Jain and Miller 27 Reference Sharhar, Normah and Fatimah 30 Reference Pan, Zhou and Gibbons 40 ). However, the results have been mixed. A meta-analysis of twenty-four case–control studies and eleven cohort studies has reported that dietary α-carotene and β-carotene intakes could significantly reduce the risk of breast cancer by 9 % (pooled relative risk 0·91, 95 % CI 0·85, 0·98) and 6 % (pooled relative risk 0·94, 95 % CI 0·88, 1·00), respectively. However, no significant association was found between dietary intakes of β-cryptoxanthin, lycopene and lutein/zeaxanthin and the risk of breast cancer( Reference Hu, Wang and Zhang 44 ). Another meta-analysis including ten prospective studies has also indicated an inverse association between the risk of breast cancer and dietary β-carotene intake, with a pooled relative risk of 0·95 (95 % CI 0·91, 0·99)( Reference Aune, Chan and Vieira 45 ). The only research conducted in China has shown an inverse association of higher dietary intakes of β-carotene, β-cryptoxanthin and lycopene with a reduced risk of breast cancer( Reference Huang, Zhang and Holman 12 ). The present findings are consistent with these studies. However, some studies have not shown the beneficial effects of specific carotenoid intake on the risk of breast cancer( Reference Terry, Jain and Miller 27 , Reference Nkondjock and Ghadirian 28 , Reference Wang, Baumgartner and Yang 30 , Reference Pan, Zhou and Gibbons 43 ).
Epidemiological studies examining the associations between carotenoid intakes and the risk of breast cancer by ER and PR status are limited, and the results are inconsistent. A pooled analysis of eighteen prospective cohort studies has suggested that dietary intakes of α-carotene, β-carotene and lutein/zeaxanthin were associated with a modestly lower risk of ER − , but not ER+, breast cancer( Reference Zhang, Spiegelman and Baglietto 46 ). However, the Women's Health Initiative Observational Study of 84 805 postmenopausal women has found that dietary intakes of α-carotene, β-carotene and lycopene were inversely associated with the risk of ER+PR+ breast cancer, but not with other breast cancer groups jointly defined by ER and PR status( Reference Cui, Shikany and Liu 21 ). In the Long Island Breast Cancer Study, β-carotene intake was found to be inversely associated with ER+PR+ and ER − PR − tumours in postmenopausal women( Reference Gaudet, Britton and Kabat 3 ). The results of the present study suggested that dietary intakes of α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin were inversely associated with all breast cancer subtypes as defined by ER and PR status.
In the present study, the protective effect of specific carotenoid intakes including α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin on the risk of breast cancer was mainly observed in pre-menopausal women. In consistent with the present study, some previous studies assessing the association by menopausal status have provided some evidence of an inverse association for pre-menopausal women( Reference Mignone, Giovannucci and Newcomb 13 , Reference Freudenheim, Marshall and Vena 19 , Reference Bohlke, Spiegelman and Trichopoulou 20 , Reference Zhang, Hunter and Forman 24 , Reference Cho, Spiegelman and Hunter 26 ), although relatively few studies have observed this relationship in postmenopausal women( Reference Gaudet, Britton and Kabat 3 , Reference Nagel, Linseisen and van Gils 22 ). The Collaborative Breast Cancer Study has found that a higher intake of β-carotene (OR 0·81, 95 % CI 0·68, 0·98) and lutein/zeaxanthin (OR 0·83, 95 % CI 0·68, 0·99) from food was associated with a statistically significant lower risk among pre-menopausal women( Reference Mignone, Giovannucci and Newcomb 13 , Reference Zhang, Hunter and Forman 24 ). Other two earlier studies( Reference Freudenheim, Marshall and Vena 19 , Reference Bohlke, Spiegelman and Trichopoulou 20 ) have also found the beneficial effects of high dietary β-carotene intake on the reduced risk of pre-menopausal breast cancer. The possible mechanism of this inverse association observed among pre-menopausal women is unclear and needs to be verified by more studies. Although the protective effect of α-carotene, β-carotene and lutein/zeaxanthin intakes on the risk of breast cancer was not found among postmenopausal women, due to the lower numbers involved in the stratified analysis, chance or low statistical power was more likely to explain some of these non-significant associations. Therefore, studies with larger sample size are needed to clarify this association.
Only ten cases and eight controls were active smokers in the present study. However, 57·2 and 42·8 % of breast cancer cases and controls were second-hand smokers. Multivariate analyses stratified by passive smoking status showed that the observed inverse association between dietary intakes of α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin and the risk of breast cancer was only confined to women exposed to SHS. So far, no previous studies have elevated the effect modification of passive smoking status on the relationship between carotenoid intake and the risk of breast cancer. However, three previous studies examined the modifying effects of active smoking status on carotenoid intake and the risk of breast cancer. The Nurses' Health Study II( Reference Cho, Spiegelman and Hunter 26 ) has shown that dietary intakes of α-carotene and β-carotene were associated with a reduced risk of breast cancer among current smokers. The Collaborative Breast Cancer Study( Reference Mignone, Giovannucci and Newcomb 13 ), a large population-based case–control study, has found a significant inverse association between dietary intakes of α-carotene, β-carotene and lutein/zeaxanthin and the risk of breast cancer among ever smokers. The Swedish Cohort study( Reference Larsson, Bergkvist and Wolk 23 ) has also shown that dietary intakes of α-carotene and β-carotene were inversely associated with the risk of breast cancer among ever smokers. These findings are consistent with a protective influence of a carotenoid-rich diet on reactive oxygen species-induced damage from tobacco smoke. It is well known that smoking causes oxidative stress and that smokers have lower plasma levels of some carotenoids. It has been suggested that during both passive and active smoking, oxidative stress is clearly exacerbated( Reference Yokus, Mete and Cakir 47 ). This might be the possible reason of the protective effect of specific carotenoid intake on the risk of breast cancer among passive smokers observed in the present study. Further studies conducted in different ethnic populations will be needed to clarify this issue.
The present study had some limitations. First, selection bias is a potential limitation in hospital-based case–control studies. The hospital-based controls may not be representative of the general population and dietary habits may be different from the general population. Another concern is that the controls recruited from hospitals may have conditions potentially related to diet. To minimise this bias, we recruited controls from several conditions with no apparent associations with a dietary cause. In addition, the high participation rate (93·3 and 94·4 % for cases and controls, respectively) and high comparability in sociodemographic factors between the two groups indicated that selection bias should not be a serious problem. Second, recall bias can be a threat to retrospective study design. The controls did not have a malignancy problem and their recall of past dietary exposures might differ from the cases. The presence of breast cancer among the case subjects might act as a stimulus, affecting the patient's perception of a possible exposure to a hypothesised risk factor( Reference Zhang, Ho and Lin 48 ). For example, breast cancer cases may have adopted a healthier eating behaviour after diagnosis which may have influenced their self-report of dietary intake. On the other hand, dietary intake of breast cancer cases may have been adversely affected by the presence of the disease. To minimise this bias, we tried to interview cases as soon as diagnosis was made. In addition, in the present study, greater effort was made to interview cases before their surgery. Moreover, food photographs with usual intake portions were provided to assist participants with quantification of intake. To improve the comparability of the recall between the cases and controls, all interviews were conducted in a hospital setting and a standardised questionnaire interview method was used among both cases and controls. Third, random measurement error in the estimation of usual intake is also of concern. Dietary intake assessed by FFQ is less precise than some other methods and may involve some measurement error. However, the results of the validation study showed that the FFQ had satisfactory reproducibility and reasonable validity. Therefore, the probability of bias arising from measurement error should be reduced in the present results.
In conclusion, dietary intakes of α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin were inversely associated with the risk of breast cancer among Chinese women. This protective effect may be more pronounced among pre-menopausal and SHS status women.
Acknowledgements
The authors very gratefully acknowledge the assistance of the student helper Juan Nie and the participation of the study subjects, without whom the study would not have been possible.
The present study was jointly supported by the New Teachers' Fund for Doctor Stations, Ministry of Education of China (no. 20100171120057), the National Natural Science Foundation of China (no. 81102188) and the Open-Lab Fund of Sun Yat-sen University in 2011 (no. KF201140). The funders had no role in the design, analysis or writing of this article.
The authors' responsibilities were as follows: L. W. conducted the data collection, analysed the data and writing of the paper; B. L. participated in the data collection; M.-X. P. and X.-F. M. were responsible for connecting and coordinating the fieldwork; Y.-M. C. provided significant advice regarding the analyses and interpretation of the data; C.-X. Z. constructed the project design, supervised and contributed to the writing of the manuscript.
The authors have no conflicts of interest to declare.








