The metabolic effects of a high-fructose diet have been widely studied in both animals and human subjects(Reference Tappy and Le1). High-fructose diet is associated with the development of at least two features of the metabolic syndrome, i.e. insulin resistance and increased plasma VLDL TAG. The latter may in turn be associated with an increased production of small, dense LDL particle with high atherogenic potential(Reference Berneis and Krauss2, Reference Aeberli, Zimmermann and Molinari3).
It has been reported by several authors that a high-fructose diet increases plasma TAG less and does not reduce insulin sensitivity in both pre-menopausal women and female rodents compared with males(Reference Melanson, Zukley and Lowndes4–Reference Song, Arikawa and Galipeau11). This may be due to a sex-specific difference in either fructose metabolism or adaptations to chronic fructose overfeeding. To our knowledge, whether the metabolism of an acute fructose load differs in females and males has not been specifically assessed. Fructose disposal relies mainly on the stimulation of carbohydrate oxidation (fructose oxidation in splanchnic organs and indirect oxidation of glucose and lactate synthesised from the fructose), gluconeogenesis, hepatic glycogen storage and hepatic de novo lipogenesis (DNL). The latter, although a quantitatively minor pathway for fructose disposal, may be closely linked to hepatic VLDL TAG production and synthesis of intra-hepatic lipids. Therefore, we hypothesised that fructose-induced stimulation of DNL may be blunted in pre-menopausal women.
To evaluate this hypothesis, we compared the metabolic fate of ingested fructose in healthy male and female human subjects. To gain insight into the pathways used for fructose metabolism, ingested fructose was labelled with 13C6 fructose, and incorporation of 13C into breath CO2, plasma glucose and plasma VLDL palmitate was monitored to evaluate total fructose oxidation, gluconeogenesis and hepatic DNL, respectively.
Subjects and methods
Subjects
Nine healthy young male (25·1 (sd 5·1) years) and nine healthy pre-menopausal female (23·8 (sd 4·8) years) subjects participated in the present study (Table 1). All the subjects were in apparent good health, had a BMI between 19 and 25 kg/m2, and were moderately physically active ( < 3 h/week). They were non-smokers, and were not taking any medications at the time of the study. None of the women were on birth control pills. Female subjects were tested during the follicular phase (days 1–10) of their menstrual cycle. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the ethical board of Lausanne University School of Biology and Medicine. Written informed consent was obtained from all the subjects.
Table 1 Anthropometric data of subjects at baseline
(Mean values and standard deviations, n 18)*
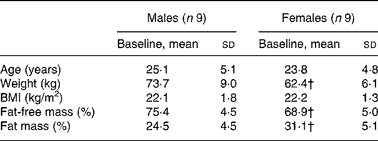
* Anthropometric data were compared in both sexes using a two-sided Wilcoxon rank sum test for unpaired values.
† Mean values were significantly different from the corresponding values in male subjects (P < 0·05).
Anthropometric and body composition measurements
At inclusion, standing height was measured using a stadiometer. Since bioelectric impedance was not available at the inclusion site, fat mass (FM) and fat-free mass (FFM) were estimated from the following skinfolds: biceps, triceps, suprailiac and subscapular(Reference Durnin and Womersley12). All the measurements were obtained on the right side, with the subject upright and relaxed. The complete set of measurements was carried out by a single operator and performed three times. Preparation of diets, fructose supplementation and the 13C fructose loads administered during the test were based on these measurements.
Body composition was measured on the day of the metabolic test by bioelectrical impedance using the BC-418 eight-contact electrode system (Tanita Corporation, Tokyo, Japan). FFM and FM were calculated using the manufacturer's equations. All the calculations pertaining to the metabolic tests were done using the value of FFM obtained by bioelectrical impedance.
Study design and diet
Each subject was studied on one occasion. During the 3 d preceding the metabolic test, subjects received a standardised, weight maintenance diet (total energy intake = 1·6 times the resting energy requirements calculated with the equation of Owen; 15 % proteins, 30 % lipids and 55 % carbohydrate (simple sugars 10 %)). During this period, all the foods were provided as pre-packed, weighed portions for each meal. Three meals and two snacks were provided each day, while subjects were asked not to consume any other food or drinks (including alcoholic beverages and coffee).
After this period of controlled diet, subjects were studied for 2 h in the post-absorptive state (time − 120–0 min) and over 6 h period after fructose ingestion (time 0–360 min).
Metabolic investigations
Subjects reported at 07.00 hours to the metabolic unit of the Lausanne University Hospital after a 10 h fast. On arrival, the subjects were asked to void, and body composition was estimated by bioelectrical impedance (Tanita Corporation). Thereafter, they rested on a bed in a semi-recumbent position until the end of the test. A catheter was inserted into an antecubital vein for collection of blood samples. The hand on the same side was kept in a thermostabilised box heated at 50°C to achieve partial arterialisation of venous blood. Another catheter was inserted into an antecubital vein of the contralateral forearm for 6,6-2H2-glucose and 2H5-glycerol infusions. Whole-body glucose and glycerol turnover was assessed using infusions of 6,6-2H2-glucose (bolus, 2 mg/kg; continuous, 40 μg/kg per min) and 2H5-glycerol (bolus, 0·097 mg/kg; continuous, 0·0097 mg/kg per min). Baseline blood and breath samples were obtained at time (T) − 120 min for the determination of background isotopic abundances. Thereafter, blood and breath samples were collected every 30 min for the next 8 h. At times 0, 120 and 240 min, the subjects ingested a lemon-flavoured drink containing 0·30 g/kg per FFM of fructose enriched with 13C6 fructose at 1·56 625 at% 13C. Energy expenditure and substrate utilisation were continuously monitored by open circuit indirect calorimetry (Deltatrac II; Datex Instruments, Helsinski, Finland). At the end of the study, urine was collected to determine urea nitrogen excretion rate. No additional foods or liquid, except water, were permitted during the test day.
Analytical procedures
Blood samples were immediately centrifuged at 4°C at 3600 rpm for 10 min, and plasma was stored in aliquots at − 20°C. Plasma glucose was measured with the glucose oxidase method (Glucose Analyser II; Beckman Instruments, Fullerton, CA, USA). Colorimetric methods were used to assess plasma concentrations of NEFA (kit obtained from Wako Chemicals, Freiburg, Germany) and TAG (kit obtained from Biomérieux Vitek, Inc., Lyon, France). Commercial RIA kits were used for the determination of plasma insulin and glucagon (Linco Research, St Charles, MO, USA). β-Hydroxybutyric acid and lactate concentrations were measured enzymatically using the kits obtained from Boehringer (Mannheim, Germany). Plasma fructose concentrations were measured by GC–MS; for this purpose, 0·1 ml of 0·55 mm-mannitol was added to 0·2 ml of plasma and vacuum dried. Thereafter, sugars were acetylated with acetic anhydride and pyridine overnight, resuspended in 0·2 ml chloroform and injected into the GC–MS apparatus (Agilent Technologies, Santa Clara, CA, USA) using a HP-5MS 5 % phenyl-methyl siloxane column. Analysis was done in electron impact mode. Ions with m/z 275 and 361 were monitored for fructose and mannitol, respectively. The ratio of m/z 361/275 was read against a standard curve prepared using pure fructose(Reference Petersen, Laurent and Yu13). Breath 13CO2 isotopic enrichment was determined by isotope ratio MS on a Tracermass C/N (SerCon Limited, Crewe, Cheshire, UK). Isotopic enrichments of plasma 6,6-2H2-glucose and 2H5-glycerol were measured by GC–MS (Agilent Technologies) as described previously(Reference Schricker, Albuszies and Kugler14, Reference Wolfe15). For the analysis of plasma 13C glucose, plasma glucose was purified by sequential anion/cation exchange chromatography using resins (AG 50 W-X8 and AG 1-X8; Bio-Rad, Richmond, CA, USA) followed by HPLC(Reference Gay, Schneiter and Schutz16). Thereafter, 13C abundance in plasma glucose was analysed by continuous flow-isotope ratio MS (Roboprep CN-Tracermass; Europa Scientific, Crewe, UK). Total lipids were extracted from the plasma, and fatty acid methyl esters were prepared from TAG fractions(Reference Bickerton, Roberts and Fielding17). The ratio of 13C:13C in the fatty acid methyl ester derivatives was ascertained using Delta Plus XP GC-combustion ratio MS (Thermo Electron Corporation, Bremen, Germany). Tricosanoic acid methyl ester was used as an isotopic enrichment standard, and a quality control sample (certified standard of eicosanoic acid and fatty acid methyl esters; Department of Geological Sciences, Indiana University, Bloomington, IN, USA) was run with each set of samples.
Calculations
Substrate kinetics and 13C glucose appearance
Endogenous glucose production (EGP), i.e. total rate of glucose appearance (Ra), was calculated from plasma 6,6-2H2-glucose isotopic enrichment using the non-steady-state equation of Steele as modified by De Bodo et al. (Reference DeBodo, Steele and Altszuler18), using a volume of distribution for glucose of 0·2 times body weight, and a pool fraction of 0·6. Total Ra glycerol was calculated from plasma 2H5-glycerol isotopic enrichment as described(Reference Romijn, Coyle and Sidossis19). The contribution of gluconeogenesis from fructose to EGP (EGP (F)) was calculated by monitoring the rate of 13C glucose appearance(Reference Proietto, Rohner-Jeanrenaud and Ionescu20) as follows:
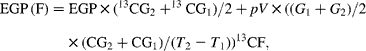
where 13CG is the isotopic enrichment of plasma glucose (atom percent (AP) excess), G is the glucose concentration, 13CF is the isotopic enrichment of oral fructose (AP excess) and T is the time. Glucose production from sources other than fructose (EGP (NF), i.e. from glycogenolysis and gluconeogenesis from lactate, amino acids and glycerol) was calculated as follows:
Substrate oxidation and energy expenditure
Net carbohydrate oxidation rates and net lipid oxidation rates were calculated from respiratory gas exchanges and urinary urea nitrogen excretion using the equations of Livesey & Elia(Reference Livesey and Elia21). Fructose oxidation was calculated as follows:
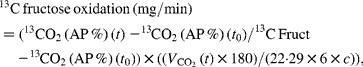
where 13CO2 (t) is the value (AP %) of the expired CO2 at time t, 13CO2 (t 0) is the value (AP %) of the expired CO2 at baseline, 13C Fruct is the value (AP %) of the ingested fructose, ![]() (l/min) is the average volume that is expired during the 30 min period, 180 is the molecular weight of fructose, 1/22·29 is the value used for conversion into moles of CO2, 1/6 is the value used for conversion into moles of fructose, c is the CO2 recovery correction factor and chosen to be 0·8(Reference Allsop, Wolfe and Burke22).
(l/min) is the average volume that is expired during the 30 min period, 180 is the molecular weight of fructose, 1/22·29 is the value used for conversion into moles of CO2, 1/6 is the value used for conversion into moles of fructose, c is the CO2 recovery correction factor and chosen to be 0·8(Reference Allsop, Wolfe and Burke22).
Non-oxidative fructose disposal (NOFD) was calculated as follows:
De novo lipogenesis
The incorporation of 13C fructose into palmitate of newly synthesised plasma TAG was assessed by monitoring the enrichment of 13C palmitate in VLDL TAG. The 13C enrichment results from fatty acid methyl ester derivatives, expressed as δ13C‰, were converted to tracer-to-tracee ratio (TTR) using the following equation:
The TTR of a baseline measurement, done before 13C-labelled fructose administration, was subtracted from each sample's TTR to account for natural abundance(Reference Chong, Fielding and Frayn23).
Statistical analysis
All parameters are expressed as means and standard deviations, except for integrative response of DNL where parameters are expressed as means with their standard errors. Post-fructose responses were calculated as incremental area under the curve (iAUC) using the trapezoidal method by subtracting the fasting value (Microsoft® Office Excel 2003; Microsoft Corporation, Redmond, WA, USA). Relative changes in hormone and substrate concentrations after fructose ingestion were calculated as 100 × (iAUC/time)/fasting value, and relative changes in substrate oxidation and glucose and glycerol kinetics after fructose ingestion were calculated as mean value (T − 30 − T 0) − fasting value/fasting value × 100. For each sex, comparisons between fasting and post-fructose values were assessed using a two-sided Wilcoxon signed rank test for paired values. The effects of fructose in males v. females were assessed by comparing the fasting parameters and integrated post-fructose parameters using a two-sided Wilcoxon rank sum test for unpaired values. Rates of glycerol appearance were markedly skewed, and were therefore log-transformed before statistical analysis. All calculations were performed with Stata 10 (Stata Corporation, College Station, TX, USA). Statistical significance was set at P < 0·05.
Results
Anthropometric and fasting metabolic parameters
Male and female participants did not differ in age or BMI. Weight and % FFM were higher, and % FM was lower in males (P < 0·05; Table 1). As shown in Table 2, baseline glucose, lactate, TAG, β-hydroxybutyrate and insulin were not significantly different, whereas NEFA tended to be lower (P = 0·08). Uric acid and glucagon concentrations were significantly higher in men (P < 0·05).
Table 2 Fasting plasma substrate and hormone concentrations of subjects at baseline
(Mean values and standard deviations, n 18)*
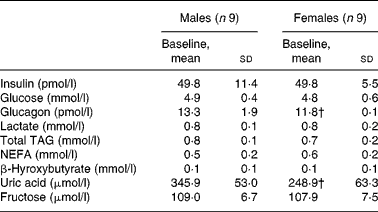
* Fasting parameters were compared in both sexes using a two-sided Wilcoxon rank sum test for unpaired values.
† Mean values were significantly different from the corresponding values in male subjects (P < 0·05).
Plasma hormone and substrate concentrations
The relative changes for fructose, glucose, glucagon, TAG and NEFA after fructose ingestion were not significantly different in males v. females, whereas the relative increases for insulin, lactate and uric acid were higher (P < 0·05) in males (Table 3).
Table 3 Relative changes in hormone and substrate concentrations after fructose ingestion, expressed as the percentage of baseline levels
(Mean values and standard deviations, n 18)*
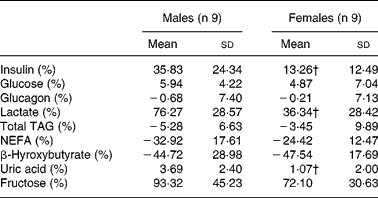
iAUC, incremental area under the curve.
* The effects of fructose in males v. females were assessed by comparing the integrated post-fructose parameters using a two-sided Wilcoxon rank sum test for unpaired values. Relative changes in hormone and substrate concentrations after fructose ingestion were calculated as 100 × (iAUC/time)/fasting value.
† Mean values were significantly different from the corresponding values in male subjects (P < 0·05).
De novo lipogenesis
Due to a technical problem with the preparation of the drink containing fructose enriched with 13C6 fructose during the first test, only eight male subjects were included for obtaining the results pertaining to DNL and 13C glucose appearance. Following fructose ingestion, the 13C enrichment of VLDL palmitate, expressed as TTR, rose significantly in males, but remained close to zero in females. The iAUC for VLDL 13C palmitate was significantly higher in males than in females (P < 0·05; Fig. 1).

Fig. 1 (a) Mean (sd) time course of the changes in 13C enrichment of VLDL palmitate in males (●, n 8) and females (○, n 9). (b) Incremental area under the curve (iAUC) of 13C palmitate tracer-to-tracee ratio (TTR) after ingestion of fructose in males (■, n 8) and females (□, n 9). Statistical significance is only indicated in the figure for differences in integrated responses between males and females. The comparison of iAUC in males v. females was assessed using a two-sided Wilcoxon rank sum test for unpaired values. * Mean values were significantly different when compared with males (P < 0·05).
Glucose and glycerol kinetics and 13C glucose appearance
Isotopic enrichments of plasma 13C glucose and of 6,6-2H2-glucose and 2H5-glycerol, which were used for the calculation of substrate kinetics, are shown in Figs. 2 and 3.

Fig. 2 Mean (sd) time course of the changes in plasma 6,6-2H2-glucose (a), 13C glucose (b) and breath 13CO2 (c) after ingestion of fructose in males (■) and females (○, n 9), except for 13C glucose and breath 13CO2 where the number of males is 8. Fructose oral loads (3 × 0·30 g/kg fat-free mass) were ingested at time 0, 120 and 240 min. MR, molar ratio; APE, atom percent excess.

Fig. 3 Mean (sd) glycerol rate of appearance (glycerol Ra) normalised for fat-free mass (FFM; a) and fat mass (FM; b) at baseline and after fructose ingestion. For each sex, comparisons between fasting and post-fructose values were assessed using a two-sided Wilcoxon signed rank test for paired values. * Mean values were significantly different when compared with baseline (P < 0·05). ■, Males; □, females.
Fasting glycerol Ra tended to be higher in females when expressed per kg FFM, but it was similar in males and females when normalised for FM (Fig. 3). Fructose ingestion significantly decreased glycerol Ra in males (baseline glycerol Ra/kg FFM × min: 3·04 v. 1·94 after fructose ingestion, P < 0·05; Fig. 3), but not in females (3·79 v. 3·19). Suppression of glycerol Ra expressed as relative decrease over the 6 h period following fructose ingestion was blunted in females ( − 41·3 (sd 15·0) in males v. − 10·2 (sd 22·9) in females; Table 3), resulting in higher post-fructose glycerol Ra (glycerol Ra/kg FFM × min: 3·19 in females v. 1·94 in males). When values were normalised for FM, glycerol Ra was still found to be decreased after fructose ingestion in males, but not in females.
Fasting glucose Ra, normalised for FFM, was slightly higher in females than in males (glucose Ra/kg FFM × min: 2·56 in males v. 3·01 in females, P < 0·05; Fig. 5). Following fructose ingestion, glucose Ra decreased slightly over time (Fig. 4). Systemic appearance of 13C glucose, corresponding to gluconeogenesis from 13C fructose, expressed as EGP (F), represented 44·01 (sd 3·2) and 41·93 (sd 2·3) % of the total glucose Ra (NS) in males and females, respectively. Glucose appearance from sources other than fructose (i.e. corresponding to glycogenolysis and gluconeogenesis from lactate, amino acids and glycerol), expressed as EGP (NF), was also similar in both sexes (Fig. 4). Fructose conversion into plasma glucose, cumulated over 6 h, represented 37·4 (sd 2·1) % of the ingested fructose in males and 28·86 % (sd 8·1) in females, P < 0·05.

Fig. 4 Mean glucose rate of appearance (glucose Ra) normalised for fat-free mass (FFM) at baseline and after fructose ingestion. Black bars represent endogenous glucose production (glucose Ra and other than fructose to endogenous glucose production (■, EGP (NF)), and white bars represent rates of exogenous (oral) fructose appearance (fructose to EGP (□, EGP (F)). The effects of fructose in males v. females were assessed by comparing fasting parameters and post-fructose parameters using two-sided Wilcoxon rank sum test for unpaired values. * Mean values were significantly different when compared with males (P < 0·05).
Net carbohydrate and lipid oxidation and energy expenditure
In basal conditions, net carbohydrate oxidation was similar in both sexes, but net lipid oxidation was higher in females (P < 0·05). Net lipid oxidation following fructose ingestion was significantly suppressed (net lipid oxidation in mg/kg FFM × min: 0·67 after fructose ingestion v. 0·78 at baseline, P < 0·05), whereas net carbohydrate oxidation increased significantly in males (net carbohydrate oxidation in mg/kg FFM × min: 2·26 after fructose ingestion v. 1·80 at baseline, P < 0·05); in females, in contrast, fructose ingestion did not significantly alter carbohydrate and lipid oxidation (Fig. 5). Fructose oxidation rate calculated from breath 13CO2 was similar in both sexes, and accounted for 42·9 (sd 3·7) and 43·0 (sd 4·5) % of the ingested fructose in males and females, respectively.

Fig. 5 Net carbohydrate oxidation (mg/kg FFM × min) and net lipid oxidation (mg/kg FFM × min) at baseline and after fructose ingestion. Values are means and standard deviations. For each sex, comparisons between fasting and post-fructose values were assessed using a two-sided Wilcoxon signed rank test for paired values. ** Mean values were significantly different when compared with baseline (P < 0·05). The effects of fructose in males (■) v. females (□) were assessed by comparing the fasting parameters using a two-sided Wilcoxon rank sum test for unpaired values. * Mean values were significantly different when compared with males (P < 0·05).
The relative change in energy expenditure after fructose ingestion, expressed as the percentage of baseline levels, was higher in males than in females and represented 3·10 (sd 2·21) and 0·06 (sd 2·63) % (P < 0·05; Table 4). The iAUC for RQ was significantly larger in males than in females (P < 0·05; Fig. 6).
Table 4 Relative changes in glucose and glycerol kinetics after fructose ingestion, expressed as the percentage of baseline levels
(Mean values and standard deviations, n 18)*
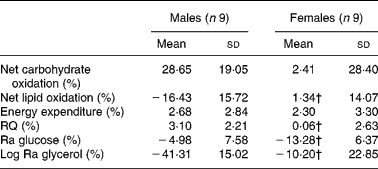
Ra, rate of glucose appearance.
* The effects of fructose in males v. females were assessed by comparing the fasting parameters using a two-sided Wilcoxon rank sum test for unpaired values. Relative changes in hormone and substrate concentrations after fructose ingestion were calculated as mean values after fructose ingestion − baseline value)/100 × baseline value.
† Mean values were significantly different from the corresponding values in male subjects (P < 0·05).
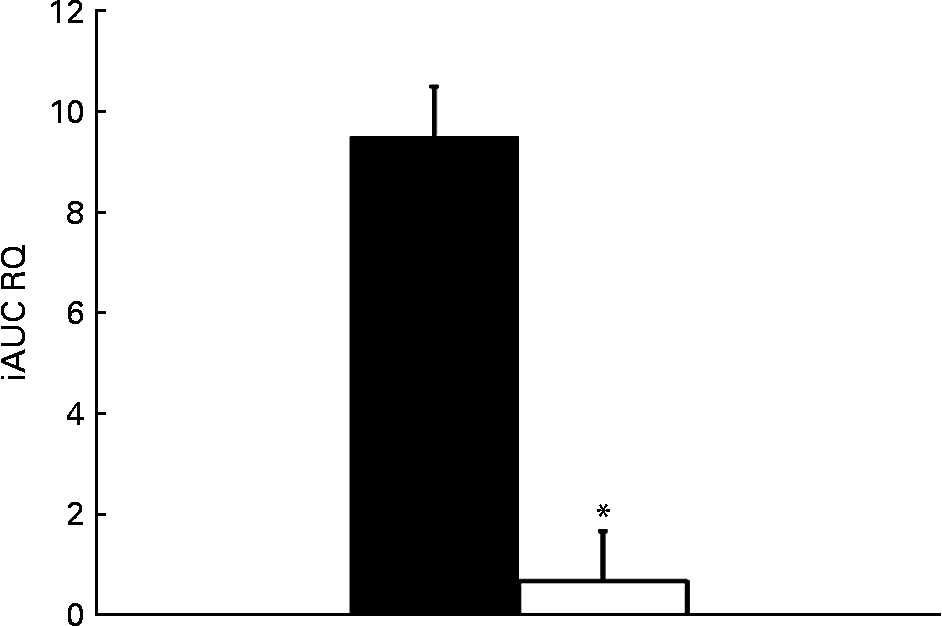
Fig. 6 Incremental area under the curve (iAUC) for RQ after fructose ingestion. The comparison of iAUC in males (■) v. females (□) was assessed using a two-sided Wilcoxon rank sum test for unpaired values. * Mean values were significantly different when compared with males (P < 0·05).
Discussion
Fructose oxidation, gluconeogenesis and glycogen storage (mainly in the liver) are known to be the major pathways of disposal of an oral fructose load in human subjects(Reference Delarue, Normand and Pachiaudi24–Reference Neese, Schwarz and Faix26). Of these, total fructose oxidation (including splanchnic fructose oxidation and indirect peripheral oxidation of glucose) was assessed by monitoring the rate of 13CO2 production, and it represented about 43–44 % of the ingested fructose load, without a difference between males and females. Conversion of fructose into plasma glucose was also evaluated by monitoring the systemic appearance of plasma 13C-labelled glucose, and it was similar in males and females. Finally, glycogen storage was not directly assessed. However, NOFD, calculated as the difference between the amount of fructose ingested and net carbohydrate oxidation, is a close approximation of glycogen synthesis, because net carbohydrate oxidation would include any fructose conversion into fat(Reference Frayn27). This was not different in males and females. Thus, the quantitatively major pathways of fructose disposal were similar in both sexes.
In previous studies, we and others have reported that short-term fructose consumption is associated with a rapid increase in fasting and post-prandial plasma TAG concentrations(Reference Chong, Fielding and Frayn23, Reference Faeh, Minehira and Schwarz28–Reference Abdel-Sayed, Binnert and Le30). Fructose is the most potent lipogenic substrate in the diet(Reference Faeh, Minehira and Schwarz28, Reference Parks, Skokan and Timlin31, Reference Stanhope and Havel32). Although fat synthesis from fructose is quantitatively minor compared with other pathways of fructose disposal, it can nonetheless have a profound impact on plasma and tissue lipids. Fructose ingestion increases hepatic malonyl-CoA(Reference Van ben Berghe33), which can increase intra-hepatic fatty acid availability both directly, by stimulating de novo fatty acid synthesis, and indirectly, by inhibiting carnitine palmitoyltransferase-1, and hence intra-hepatic fatty acid oxidation. In a previous study, we have indeed observed that fractional hepatic DNL was positively correlated with plasma VLDL TAG concentrations after the ingestion of a short-term high-fructose diet. Furthermore, inhibition of DNL by a supplementation with long-chain PUFA of marine origin led to a proportionate suppression of VLDL TAG(Reference Faeh, Minehira and Schwarz28). Based on these observations, it appears reasonable to speculate that fructose-induced DNL is instrumental in eliciting at least some of the adverse metabolic effects of fructose. This raised the hypothesis that fructose may stimulate hepatic DNL to a lesser extent in females than in males. The present results support this hypothesis. We indeed observed a significant incorporation of 13C-labelled palmitate in VLDL TAG, together with a significant suppression of whole-body lipid oxidation in males, whereas these two effects were conspicuously absent in females. This strongly suggests that females had less fructose-induced DNL. Moreover, we observed a much larger increase in the RQ of males after fructose ingestion, consistent with the suppression of lipid oxidation and an increased conversion of carbohydrate into fat(Reference Frayn27, Reference Brown, Dulloo and Yepuri34). Differences in fatty acid synthesis between males and females did not, however, mirror changes in fructose oxidation, gluconeogenesis or glycogen storage, most likely because only a small amount of fructose is converted into fatty acids.
In addition to stimulating more DNL in males than in females, fructose ingestion also differentially affected whole-body lipid metabolism in males v. females. Fructose ingestion is known to acutely suppress plasma NEFA and whole-body lipid oxidation. This is best explained by the significant, albeit modest stimulation of insulin secretion elicited by fructose, which efficiently inhibits adipose tissue lipolysis(Reference Tappy, Randin and Felber35). Glycerol rate of appearance, which, in the absence of exogenous fat metabolism, mainly reflects adipose tissue lipolysis, was more suppressed in males following fructose ingestion. Although part of the difference may be due to the physiologically higher body fat content of females, the glycerol appearance rate normalised for FM remained less suppressed after fructose ingestion in females. This may be explained by the somewhat higher (but NS) insulin response elicited by fructose in males.
The present results point to differences in lipid metabolism in adipose tissues and in the liver of females compared with males. The mechanisms involved in this difference have not been specifically assessed in the present study, and can only be speculated. Several observations strongly support the role of oestrogen in this process. Thus, a lesser increase in plasma TAG after fructose ingestion is observed in pre-menopausal women, but not in post-menopausal women(Reference Stanhope, Griffen and Bair5). In rodents, sex differences in metabolic responses to fructose can be eliminated by oophorectomy and restored by oestrogen substitution(Reference Bantle, Raatz and Thomas6, Reference Couchepin, Le and Bortolotti7). Our observation points to a dual effect of oestrogens, which stimulate adipose tissue lipolysis while decreasing hepatic DNL. The effect of oestrogen on adipose tissues has indeed been documented by several studies: adipocytes from female mice are known to have increased lipogenic and lipolytic rates compared with those from male mice(Reference Pujol, Rodriguez-Cuenca and Frontera36–Reference Cooke and Naaz39). Human in vivo experiments have also demonstrated that oestrogens increase fatty acid availability and oxidation(Reference Halkes, van Dijk and Verseyden40, Reference Blaak41). In post-menopausal patients, oestrogen replacement therapy reduced the expression of key genes involved in fatty acid synthesis and fat storage, including acetyl-CoA carboxylase, fatty acid synthase and PPARγ(Reference Lundholm, Zang and Hirschberg42). Regarding the hepatic effects of oestrogens, to our knowledge, no data are available in human subjects. Oestrogen treatment of female rats, however, reduced hepatic acetyl-CoA carboxylase activity(Reference Diamant, Neuman and Shafrir43). Furthermore, oophorectomy decreased the expression of PPARα and increased that of sterol regulatory element binding protein-1c, and increased liver TAG content, consistent with a switch towards fat synthesis upon oestrogen withdrawal(Reference Paquette, Wang and Jankowski44). Oestrogen treatment also reversed hepatic steatosis in male rats made deficient in aromatase(Reference Hewitt, Pratis and Jones45).
The present study has several limitations which have to be kept in mind. First, hepatic DNL was qualitatively assessed by monitoring the incorporation of 13C administered as 13C6 fructose into VLDL palmitate over time. This is a relatively crude method, which does not allow a quantitative assessment of fatty acid synthesis due to the absence of steady-state conditions and of a simultaneous measurement of VLDL kinetics. Secondly, this method provides an estimate of fractional hepatic DNL, i.e. the fraction of VLDL palmitate issued from de novo synthesis; it is therefore possible that this value was somewhat underestimated in females than in males due to a higher NEFA flux in females. Finally, the fructose load administered was normalised for FFM because the latter is the best predictor of whole-body metabolism. However, this led to a significantly higher administration of fructose per kilogram predicted liver weight in males than in females. This relative hepatic fructose overloading may explain the higher lactate and uric acid responses in males; it may also contribute to a more important stimulation of hepatic DNL in males.
Conclusion
The present data indicate that oral doses of fructose differentially affect hepatic DNL, adipose tissue lipolysis and lipid oxidation in males and females. This may be due to the effects of female sex hormone on hepatic and adipose metabolism. However, it remains to be assessed whether this sex difference in fructose metabolism confers a significant protection against the potentially harmful effects of fructose in women.
Acknowledgements
We thank the staff of the Département de Physiologie de Lausanne and of the Cardiomet Clinical Investigation Center for their excellent assistance, and all the volunteers for their participation. The present study was supported by grant no. 310030_121995 from the Swiss National Foundation for Science to L. T.; B. A. F. and K. N. F. were supported by the Integrated Project ‘Hepatic and adipose tissue functions in the metabolic syndrome’ and by the European Commission LSHM-CT-2005-018734 contract no. 018734. The authors' contributions are as follows: C. T., D. J.-D., G. C., K.-A. L. and L. T. designed the study and performed the clamp experiments. P. S. and B. A. F. performed incorporation of 13C into palmitate and plasma glucose and breath CO2 measurement. C. T., D. J.-D., V. L. and L. T. analysed the data. C. T. wrote the draft manuscript, and D. J.-D., V. L., B. A. F., G. C., K.-A. L., M. B., K. N. F., P. S. and L. T. reviewed and edited the manuscript. None of the authors has any conflict of interest.












