Iron homeostasis is maintained by changes in the efficiency of intestinal absorption and recycling of haem iron by macrophages in the reticuloendothelial system. Hepcidin, a twenty-five-amino-acid peptide produced from a pre-propeptide of eighty-four amino acids synthesised in the liver (Krause et al. Reference Krause, Neitz, Mägert, Schulz, Forssmann, Schulz-Knappe and Adermann2000; Park et al. Reference Park, Valore, Waring and Ganz2001; Pigeon et al. Reference Pigeon, Ilyin, Courselaud, Leroyer, Turlin, Brissot and Loreal2001), reduces iron absorption in mucosal epithelial cells (Laftah et al. Reference Laftah, Ramesh, Simpson, Solanky, Bahram, Schumann, Debnam and Srai2004) and iron release from macrophages (Nicolas et al. Reference Nicolas, Bennoun, Devaux, Beaumont, Grandchamp, Kahn and Vaulont2001, Reference Nicolas, Bennoun and Porteu2002) in response to high iron levels and inflammation (Nemeth et al. Reference Nemeth, Valore, Territo, Schiller, Lichtenstein and Ganz2003). Hepcidin influences iron absorption through direct binding to ferroportin at the basolateral membrane, leading to decreased export of cellular iron (Nemeth et al. Reference Nemeth, Tuttle, Powelson, Vaughan, Donovan, Ward, Ganz and Kaplan2004a). Reduced iron uptake into epithelial cells through down-regulation of divalent metal transporter 1 (DMT1) expression (Yamaji et al. Reference Yamaji, Sharp, Ramesh and Srai2004) has also been reported as a possible mode of action.
Hepcidin plays a major role in iron homeostasis, but full characterisation of its role in healthy humans and patients has been hindered by the absence of analytical methods to quantify circulating levels in the blood. Quantification of mRNA in the liver has been undertaken using reverse transcription and the polymerase chain reaction (Bridle et al. Reference Bridle, Frazer and Wilkins2003; Gehrke et al. Reference Gehrke, Kulaksiz, Herrmann, Riedel, Bents, Veltkamp and Stremmel2003), and polyclonal antibodies to refolded synthetic hepcidin have been produced and used for quantification in urine (Nemeth et al. Reference Nemeth, Valore, Territo, Schiller, Lichtenstein and Ganz2003). Attempts to produce correctly folded synthetic hepcidin have proved difficult because the sequence contains eight cysteine residues that constrain the hepcidin molecule in a hairpin structure (Hunter et al. Reference Hunter, Fulton, Ganz and Vogel2002). Hepcidin in urine has also been quantified using mass spectrometry methods (Kemna et al. Reference Kemna, Tjalsma, Laarakkers, Nemeth, Willems and Swinkels2005; Liang et al. Reference Liang, Clarke, Chan and Zhang2006; Tomosugi et al. Reference Tomosugi, Kawabata, Wakatabe, Higuchi, Yamaya, Umehara and Ishikawa2006).
Prohepcidin, the sixty-amino-acid product of cleavage of the signal peptide from the hepcidin precursor, is expressed at the basolateral membrane domain of hepatocytes and is found in blood (Kulaksiz et al. Reference Kulaksiz, Gehrke, Janetzko, Rost, Bruckner, Kallinowski and Stremmel2004). Serum prohepcidin concentrations are significantly lower in patients with hereditary haemochromatosis compared to healthy control subjects (Kulaksiz et al. Reference Kulaksiz, Gehrke, Janetzko, Rost, Bruckner, Kallinowski and Stremmel2004), increase with declining kidney function (Taes et al. Reference Taes, Wuyts, Boelaert, De Vriese and Delanghe2004), and are correlated with haematocrit in chronic haemodialysis patients (Hsu et al. Reference Hsu, Chiang, Chien and Hung2006), but it is currently unclear whether serum prohepcidin is a measure of active hepcidin or a non-functional precursor (Brookes et al. Reference Brookes, Sharma, Tselepis and Iqbal2005). The relationship between circulating prohepcidin concentration and iron absorption in humans has not yet been explored. The aims of the research reported in the present paper were to examine the association between prohepcidin and iron status in subgroups of the population with differing iron requirements and metabolism (men carrying HFE mutations and wild-type controls, hereditary haemochromatosis patients undergoing phlebotomy treatment, and pregnant women), and to determine the extent to which serum prohepcidin concentration predicts iron absorption in a group of healthy men.
Methods
Serum prohepcidin concentration was measured in samples collected in three human studies.
Study 1: C282Y heterozygotes and wild-type controls
Five hundred and fifty-six men from the Norwich (UK) area, aged 40 years and over, were recruited to studies investigating links between dietary iron, genotype and health (Roe et al. Reference Roe, Heath and Oyston2005). A 10 ml blood sample was taken and the HFE genotype was determined by the Molecular Genetics Department of the Norfolk and Norwich University Hospital, UK (Willis et al. Reference Willis, Scott, Jennings, Smith, Bukhari and Wimperis2002). Seventy-four C282Y/wild heterozygote and 325 wild-type men were identified. Subjects carrying the H63D or S65C mutation of the HFE gene were not included in the studies. Volunteers with a diagnosed medical condition likely to affect diet or gastrointestinal function (e.g. diabetes, coeliac disease) were excluded. One-hundred and thirty-eight of these men were recruited to an iron status study, and thirty to an iron absorption study as described later.
Data for thirty (fifteen wild/wild controls, fourteen C282Y/wild heterozygote) men recruited to take part in an investigation of the effect of HFE genotype on iron absorption (Roe et al. Reference Roe, Heath and Oyston2005) were used to determine the association between serum prohepcidin concentration and iron absorption in healthy men. Meals (high iron bioavailability and fortified cereal products) were labelled extrinsically with stable isotopes of non-haem iron, and absorption was measured from the isotopic enrichment of red blood cells 14 d post-administration using the haemoglobin incorporation technique (Roe et al. Reference Roe, Heath and Oyston2005).
Data for 138 (forty-seven C282Y/wild heterozygotes and ninety-one wild/wild controls) men recruited to take part in an investigation of the effect of HFE genotype, diet and lifestyle on iron status were used to determine the association between serum prohepcidin concentration and iron status in healthy men carrying HFE mutations. Participants attended the Human Nutrition Unit at the Institute of Food Research (UK) on three occasions over the course of one week to provide fasting blood samples for biochemical analysis. Serum ferritin, serum iron, total iron-binding capacity, transferrin saturation, C-reactive protein and soluble transferrin receptor (sTfR) were determined on each of the three days. Haemoglobin and HFE genotype were determined on day 1 only. All analyses, except sTfR and HFE genotype, were determined by the Chemical Pathology Department of the Norfolk and Norwich University Hospital, UK. sTfR concentration was measured in EDTA plasma using a commercially available ELISA kit (Quantikine IVD Soluble Transferrin Receptor ELISA, R&D Systems Europe Ltd, Oxon, UK).
Study 2: Haemochromatosis patients
Six patients with a clinical diagnosis of hereditary haemochromatosis (five male, one female) were recruited to take part in a study on serum non-transferrin iron-binding compounds in chronic iron overload. All six patients were C282Y homozygotes. Up to five 100 ml samples of whole blood were collected from each patient during routine clinical venesection over a period of 6 months. Serum ferritin, serum iron, total iron-binding capacity and transferrin saturation were determined by the Chemical Pathology Department of the Norfolk and Norwich University Hospital, UK.
Study 3: Pregnant women
Healthy pregnant women aged 18–40 years (n 13) were recruited to a study investigating the effects of iron supplements on zinc and copper metabolism. A blood sample was taken between 10 and 14 weeks of pregnancy to exclude women whose biochemical and haematological indices fell outside the normal range, were anaemic (haemoglobin < 10·8 g/dl), or had low iron stores (serum ferritin < 23 μg/l). Blood donation during the previous 6 months was also in the exclusion criteria. The study was a randomised single-blind placebo-controlled study with subjects being given a daily supplement of 100 mg iron as ferrous gluconate or placebo from 16 weeks' gestation until delivery. Fasting blood samples were taken at 16, 24 and 34 weeks of pregnancy and iron status determined from serum ferritin, transferrin saturation and sTfR measurements. Serum ferritin concentrations were determined in triplicate using an in-house ELISA (Flowers et al. Reference Flowers, Kuizon, Beard, Skikne, Covell and Cook1986). Transferrin saturation was calculated from direct measurements of serum iron concentration (Carter, Reference Carter1971) and total iron-binding capacity using methods automated on a Cobas Mira autoanalyser (Roche, Switzerland). sTfR concentration was determined in serum as described for study 1.
Ethics
All studies were approved by the Norwich Local Research Ethics Committee (LREC) and written informed consent was obtained from all subjects. Ethical permission for the analysis of prohepcidin from stored samples was obtained retrospectively from the LREC.
Measurement of serum prohepcidin
Serum samples were stored at − 80°C and allowed to return to room temperature before analysis. Samples were measured using the DRG Diagnostics Hepcidin Prohormone ELISA (Immunodiagnostic Systems Ltd, Boldon, Tyne & Wear, UK) according to the manufacturer's instructions. Samples, controls and standards (50 μl) were measured in duplicate, and were added together with fixed quantities of assay buffer (50 μl) and hepcidin(28–47)–biotin conjugate (50 μl) to anti-hepcidin(28–47) antibody-coated microtitre plate wells. After incubation at room temperature and washing, streptavidin–horse radish peroxidase conjugate was added to the wells. Following a further incubation and washing step, enzyme substrate was added, incubated and then stop solution added prior to determination of absorbance of each well at 450 nm. Standard curves were plotted using a four-parameter logistic fit (Prism 4, GraphPad Software Inc., San Diego, CA, USA). Sample and control concentrations were determined from these plots. In addition to the high- and low-level internal kit controls, two further serum sample controls were used to assess inter-assay variation.
Statistics
The relationship between serum prohepcidin, iron status and genotype, and the relationship between serum prohepcidin concentration and iron absorption in study 1 (C282Y heterozygote and wild-type controls), and the relationship between serum prohepcidin and iron status in study 2 (haemochromatosis patients) were examined using analysis of variance (ANOVA) models with backwards elimination. A repeated measures model was also used in study 2 to investigate the relationship between serum prohepcidin and iron status following phlebotomy treatment. The relationship between serum prohepcidin, iron status and iron supplementation in study 3 (pregnant women) was examined using a repeated measures model. The statistics package R was used for all analyses (R Development Core Team, 2003).
Results
Study 1: C282Y heterozygotes and wild-type controls
Iron absorption
Mean serum prohepcidin concentration was 164 ng/ml (sd 68, range 78–315) in wild-type subjects and 201 ng/ml (sd 72, range 106–360) in C282Y heterozygotes. The relationship between serum prohepcidin concentration and absorption of iron added to breakfast cereals is shown in Fig. 1. ANOVA showed no significant association between iron absorption and serum prohepcidin concentration. Explanatory variables associated with iron absorption were serum ferritin (P < 0·001) and total iron-binding capacity (P = 0·008). Serum ferritin had a negative association (r = − 0·65) with iron absorption and total iron-binding capacity had a positive association (r = 0·46).
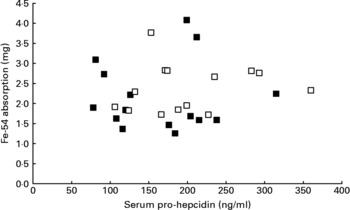
Fig. 1 Relationship between serum prohepcidin and absorption of iron added to cereal products in wild-type (■) and C282Y heterozygotes (□).
Iron status
Mean serum prohepcidin concentration was 208 ng/ml (sd 122, range 56–902) in wild-type subjects and 225 ng/ml (sd 109, range 55–707) in C282Y heterozygotes. Serum prohepcidin, haemoglobin, serum ferritin, plasma transferrin receptor and serum iron were not significantly different between genotype groups but transferrin saturation was significantly higher in C282Y heterozygotes (31·2 (sd 8·6)) than in wild-type controls (27·1 (sd 7·4)). Analysis of variance showed no association between serum prohepcidin concentration and indices of iron status (haemoglobin, serum ferritin, plasma transferrin receptor, serum iron and transferrin saturation), or C282Y genotype. There is a substantial body of evidence demonstrating that C282Y heterozygotes do not accumulate significant additional iron compared to wild-type individuals (Heath & Fairweather-Tait, Reference Heath and Fairweather-Tait2003) and this is supported by our iron status and absorption data. The study population therefore represents variability in a phenotypically heterogeneous group.
Mean intra-individual CV of the three samples for each individual was 12·6 %, indicating that fasting serum prohepcidin concentration is not subject to large day-to-day variation. Mean intra-individual CV for serum ferritin was 11·3 %. Mean inter-assay CV of the serum control samples was 14·3 %.
Study 2: Haemochromatosis patients
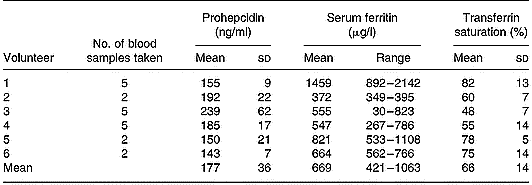
Serum prohepcidin concentration in haemochromatosis patients was 177 ng/ml (sd 36) (Table 1) and was not significantly different to that in C282Y heterozygotes (mean 225 ng/ml, sd 109) or wild-type controls (mean 208 ng/ml, sd 122). There was no correlation between serum prohepcidin and serum ferritin (R 2 = 0·14) or transferrin saturation (R 2 = 0·01).
Table 1 Serum prohepcidin and ferritin concentrations in haemochromatosis patients undergoing phlebotomy treatment (study 2)
Study 3: Pregnant women
The iron status of the pregnant volunteers at 16, 24 and 34 weeks' gestation is presented in Table 2. Mean serum prohepcidin concentration in pregnant women was 159 (sd 59) ng/ml. Serum ferritin concentrations decreased during the course of pregnancy in both the placebo and supplement groups, with a greater decline in the placebo group, but there were no significant changes in serum prohepcidin concentration. ANOVA showed that serum prohepcidin concentration was not associated with any of the iron status measures, week of gestation or iron supplementation. The interaction term supplement group:week of gestation was significantly associated with all four iron status indicators but not prohepcidin concentration.
Table 2 Serum prohepcidin and iron status in pregnant women given iron (100 mg Fe/day as ferrous gluconate) or placebo by week of gestation (study 3)
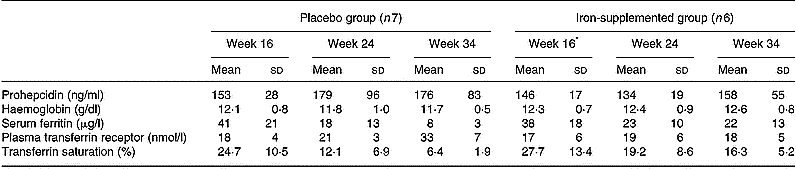
* Note that the week 16 blood sample was taken before iron supplementation had started.
Discussion
There is a growing body of evidence supporting the role of hepcidin as a key regulator of iron metabolism, but the molecular mechanisms by which it interacts with other proteins involved in iron metabolism are unclear. Despite the enormous interest in the role of hepcidin, lack of available methods for quantifying circulating hepcidin in clinical samples has restricted the quantity and quality of published data. Nemeth et al. (Reference Nemeth, Valore, Territo, Schiller, Lichtenstein and Ganz2003) measured hepcidin in urine with a Western blotting assay using antibodies raised to a synthetic hepcidin and showed a significant correlation between urinary hepcidin and serum ferritin concentrations in subjects with ferritin concentrations ranging from 10 to 10 000 ng/ml. Patients with anaemia of inflammation, diagnosed by elevated serum ferritin and clinical history, had elevated urinary hepcidin excretion compared with healthy subjects and patients with iron deficiency anaemia or controlled haemochromatosis. Nemeth et al. (Reference Nemeth, Rivera, Gabayan, Keller, Taudorf, Pedersen and Ganz2004b) used the same antibodies in a dot blot assay and showed that urinary hepcidin excretion increased rapidly in response to IL-6 infusion with a corresponding decrease in serum iron, and also showed that urinary hepcidin increased following a 65 mg oral dose of bioavailable iron. Dallalio et al. (Reference Dallalio, Fleury and Means2003) used an antibody raised to hepcidin-25, which bound to a 9-kDa protein, to quantify hepcidin in human serum using a Western blot assay. Serum hepcidin concentration was significantly correlated with serum ferritin in fifty-five patients undergoing serum ferritin analysis and in thirty-seven anaemic patients undergoing bone marrow investigation. There were no significant differences in serum hepcidin concentration in patients with anaemia of differing aetiology.
An ELISA using prodomain antiserum has been developed (Kulaksiz et al. Reference Kulaksiz, Gehrke, Janetzko, Rost, Bruckner, Kallinowski and Stremmel2004) and has been used in recent publications reporting serum prohepcidin concentrations in a range of subjects (Ezeh et al. Reference Ezeh, Ugochukwu, Weinstein and Okpala2005; Kulaksiz et al. Reference Kulaksiz, Theilig, Bachmann, Gehrke, Rost, Janetzko, Cetin and Stremmel2005). The mean serum prohepcidin concentration (214 ng/ml, range 56–902 ng/ml) found in the present study in healthy men aged 40 years and over was higher than that reported in twenty-six healthy male and female subjects (mean 106, range 52–153 ng/ml) (Kulaksiz et al. Reference Kulaksiz, Gehrke, Janetzko, Rost, Bruckner, Kallinowski and Stremmel2004) and in thirty healthy male and female subjects (mean 84 (sd 40)) (Ezeh et al. Reference Ezeh, Ugochukwu, Weinstein and Okpala2005). The present data show no significant correlation between serum prohepcidin and serum ferritin or serum iron. Kulaksiz et al. (Reference Kulaksiz, Gehrke, Janetzko, Rost, Bruckner, Kallinowski and Stremmel2004) also failed to find any association between serum prohepcidin and iron status measures, and additionally reported a lack of correlation between serum and urine prohepcidin concentration, although the concentration in urine was higher than in serum (Kulaksiz et al. Reference Kulaksiz, Theilig, Bachmann, Gehrke, Rost, Janetzko, Cetin and Stremmel2005).
Serum prohepcidin concentrations in blood samples from hereditary haemochromatosis patients (Table 1) and pregnant women (Table 2) were not significantly different from those of men with normal iron stores and were not related to differences in iron stores. Even in haemochromatosis patients undergoing phlebotomy treatment, the range of serum prohepcidin concentration was small, despite the large differences in serum ferritin concentrations. Similarly, serum prohepcidin concentration in pregnant women who were not supplemented with iron did not change in response to significant reductions in serum ferritin. In contrast to the present findings, Kulaksiz et al. (Reference Kulaksiz, Gehrke, Janetzko, Rost, Bruckner, Kallinowski and Stremmel2004) reported that haemochromatosis patients had significantly lower serum prohepcidin concentrations than controls, whereas renal patients undergoing haemodialysis had significantly higher concentrations. However, in support of the present observations that serum prohepcidin is relatively consistent across different physiological states, Ezeh et al. (Reference Ezeh, Ugochukwu, Weinstein and Okpala2005) demonstrated that serum prohepcidin concentrations in patients with sickle cell anaemia with abnormally high serum ferritin concentration were no different to controls.
Hepcidin concentrations would be expected to change in response to changes in iron balance and to increase in response to inflammation. Schümann et al. (Reference Schümann, Kroll, Weiss, Frank, Biesalski, Daniel, Friel and Solomons2005) monitored serum prohepcidin concentration in three healthy volunteers over a period of 13 d and reported concentrations in the range 150–350 ng/ml when receiving a daily oral dose of 120 mg iron. Such a large dose of oral iron may be expected to significantly increase hepcidin expression but concentrations were relatively stable, although significantly different to baseline measurements over the three days prior to iron supplementation. However, in contrast to the present, and other published, data, fasting baseline values were reported to be extremely low (below detectable limits ( < 4 ng/ml) in two subjects and 22 ng/ml in the other). Day-to-day variation in fasting serum prohepcidin within individuals in our study was low and was comparable to serum ferritin, which is considered to be relatively stable.
There was no association between serum prohepcidin concentration and efficiency of iron absorption (Fig. 1). When the reasons for inter-individual variation in absorption were explored, serum ferritin and total iron-binding capacity were the only variables significantly associated with iron absorption.
The present results and previously published data have not shown consistent changes in serum or urinary prohepcidin concentrations and cast doubt over the use of prohepcidin as a surrogate marker for hepcidin. It is possible that the functional N-terminal antibody used to detect serum prohepcidin may determine a non-functional precursor of hepcidin rather than the active form (Brookes et al. Reference Brookes, Sharma, Tselepis and Iqbal2005). The identity of hepcidin immunoreactive bands on Western blots has also been questioned (Walker et al. Reference Walker, Partridge, Srai and Dooley2004) and there seem to be discrepancies in the molecular masses of proteins determined by Western blots compared to masses predicted by sequences. Further development of methods for quantification of circulating functional hepcidin will be necessary to enable greater understanding of this important protein.
In conclusion, the present results show that fasting serum prohepcidin concentrations are not associated with iron stores and are not significantly different in hereditary haemochromatosis patients, pregnant women, and healthy men. Moreover, differences in serum prohepcidin do not explain inter-individual variation in iron absorption in healthy men. Consequently, it seems that serum prohepcidin does not have a functional role in iron homeostasis and is therefore not a useful biomarker. There is an urgent need to develop a reliable and accurate method for quantifying circulating forms of hepcidin that can be used in both clinical and research settings.
Acknowledgements
The authors gratefully acknowledge the support of the UK Food Standards Agency, the Biotechnology and Biological Sciences Research Council, the New Zealand Health Research Council (Fellowship to A.-L.M.H.) and the European Commission (QLK1-1999-00 337). The authors thank the volunteers for participating in the studies and Catherine Macrow, Sarah Oyston and Wendy Hollands (Institute of Food Research, UK) for assistance with participant recruitment, and Aliceon Blair, Linda Oram, Lesley Maloney (Human Nutrition Unit, Institute of Food Research, UK), G. Pout and E. McClagish (Norfolk and Norwich University Hospital NHS Trust, UK) for clinical assistance.





